Physicists Start To Pin Down How Stars Forge Heavy Atoms

Experiments in Michigan are unraveling how elements heavier than iron are made.
Mark Belan/Quanta Magazine
Introduction
The Facility for Rare Isotope Beams (FRIB) may not glitter quite like the night sky, plunked as it is between Michigan State University’s chemistry department and the performing arts center. Inside, though, the lab is teeming with substances that are otherwise found only in stars.
Here, atomic nuclei accelerate to half the speed of light, smash into a target and shatter into smithereens. The collisions create some of the same rare, unstable isotopes that arise inside stars and which, through a sequence of further reactions, end up as heavy elements.
FRIB scientists have been re-creating the recipe.
“People like to do DNA tests to see where their ancestors came from,” said Artemis Spyrou, a nuclear astrophysicist at FRIB. “We’re doing the same with our planet and solar system.”
Scientists have a solid understanding of how stars forge the elements on the periodic table up to iron. But the processes that give rise to heavier elements — zinc, lead, barium, gold and the rest — are more elusive.
Now, tangible results have emerged in a field replete with postulates and presumptions. The FRIB lab is currently replicating one of the three main processes by which heavy elements are thought to form, and homing in on where this “intermediate neutron-capture process,” or i-process, occurs.
The lab also plans to re-create one of the other two processes as well, the one that yields “jewelry shop elements” such as platinum and gold.
“This is a big, big jump forward in understanding how isotopes form. Then we can go backward and find the astrophysical sites with the right conditions,” said John Cowan, who first theorized about the i-process as a graduate student in the 1970s. “FRIB is doing some pioneering work.”
Building the Elements
Some 13.8 billion years ago, the newborn universe was a scorching soup of elementary particles, freshly forged in the Big Bang. As the cosmos cooled and expanded, these specks combined to form subatomic particles such as protons and neutrons, which combined to form hydrogen, helium and lithium — the first and lightest elements — during the universe’s first three minutes. It would take another couple hundred million years for these elements to clump together into larger bodies and birth stars.
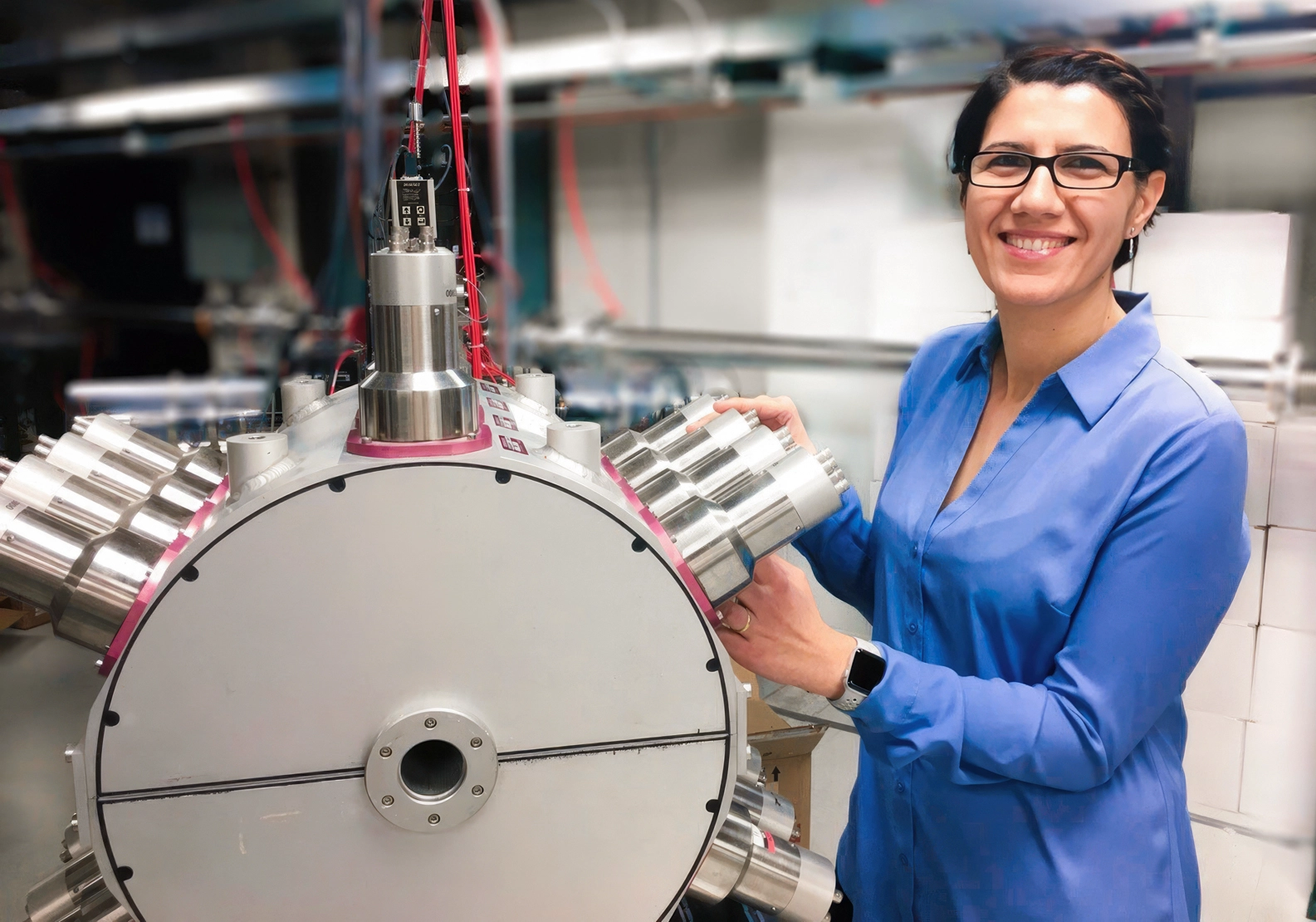
Artemis Spyrou, a physicist at the Facility for Rare Isotope Beams (FRIB) and Michigan State University, poses with the Summing Nal (SuN) detector at FRIB, a gamma-ray detector used to measure decaying isotopes.
Courtesy of Facility for Rare Isotope Beams
Once stars lit up the cosmos, the universe grew chemically richer. In a star’s hot, dense core, atomic nuclei smash into each other with immense force, fusing to form new elements. When hydrogen nuclei (which have one proton apiece) fuse, they form helium; three of those fuse into carbon, and so on. This nuclear fusion releases heaps of energy that presses outward, preventing the star from collapsing under the pressure of its own gravity. As a massive star ages, it fuses increasingly heavy elements, moving up the periodic table. That is, until it gets to iron.
At that point, further fusion doesn’t release energy; it absorbs it. Without new energy from fusion, the star’s death becomes imminent. Its core contracts inward, and a shock wave blasts everything else outward — creating a supernova.
For everything past iron on the periodic table, a different origin story is needed.
In the 1950s, physicists came up with one: “neutron capture.” In this process, nuclei collect neutral, free-floating subatomic particles called neutrons. As these glom on, the nucleus becomes an unstable version of itself — a radioactive isotope. Balance is restored when its excess neutrons transform into positively charged protons in a process called beta decay. Gaining a proton turns the nucleus into the next element on the periodic table.
Mark Belan/Quanta Magazine
To reach its final form, an atomic nucleus typically moves through a string of different radioactive isotopes, collecting more and more neutrons as it goes.
At first, scientists thought there were only two pathways for atoms to travel in order to grow big. One is slow and the other rapid, so they’re called the s-process and the r-process.
In the s-process, an atomic nucleus spends thousands of years sporadically capturing neutrons and decaying before reaching its final, stable destination. It’s thought to occur in extra-luminous, inflated stars called red giants, particularly during a phase when they’re known as asymptotic giant branch stars. (One day our own star should turn into such a red giant.) As the giant teeters on the brink of death, its inner layers mix to create just the right neutron-rich environment for the s-process to unfold.
Meanwhile, the r-process lasts only seconds. It requires an environment with a far denser population of neutrons, such as a neutron star — the ultra-dense, neutron-packed core of a dead star. The r-process probably occurs when two neutron stars collide.

U Camelopardalis is an asymptotic giant branch star, the kind of red giant that hosts the s-process. Every couple thousand years, the helium shell surrounding the star’s core begins to burn, encasing the star in a bubble of gas, as seen in this image by the Hubble Space Telescope. These helium flashes are a candidate setting of the i-process.
ESA/Hubble, NASA and H. Olofsson (Onsala Space Observatory)
The s-process and r-process forge many of the same final elements, but in different proportions. The former will create more barium, for example, while the latter creates lots of europium. These elements fly out into the interstellar medium when the star dies and are incorporated into a new generation of stars. Astronomers can observe the new stars and, by the elements they find in them, infer what processes produced their raw materials.
For decades, the scientific consensus was that the slow and rapid processes were the only ways to produce heavy elements. Eventually, though, scientists began to think about a middle path.
An In-Between Process
Cowan dreamt up an intermediate neutron-capture process during his graduate work at the University of Maryland in the 1970s. While studying red giant stars for his thesis, he proposed possible nuclear reaction pathways and neutron densities that didn’t fit the s- or r-process. “But it was just an idea then,” he said.
Then, in the early 2000s, cracks appeared in the s-versus-r dichotomy. Typically, stars offer hints that either the slow or rapid process occurred sometime before their birth, depending on which heavy elements are more abundant in them. Astronomers tend to find clear signatures of one process or the other in “carbon-enhanced, metal-poor” stars, ancient stars that have just one-thousandth the iron of our sun but more carbon than usual relative to iron. But when they studied some of these stars in the Milky Way’s outskirts, they saw element abundances that didn’t match the fingerprints of either process.
“It left people scratching their heads,” said Falk Herwig, a theoretical astrophysicist at the University of Victoria.
Mark Belan/Quanta Magazine
Herwig began to think of new scenarios. One candidate was a “born-again” red giant star. On rare occasions, the burnt-out corpse of a red giant, called a white dwarf, can reignite when the helium shell surrounding its core starts to fuse again. Helium burning in other, non-resurrected red giants might fit the bill, too, as long as the stars are metal-poor.
Another possibility: A white dwarf siphons off material from a companion star. If it accumulates enough mass this way, it can start to fuse helium. The flash of energy is so powerful that it can cause the white dwarf to spew its outer layers, ejecting new elements along the way, Herwig thought.
When he presented his idea at a conference in 2012, Cowan was in the audience. “He came up to me and said, ‘I had this paper in the 1970s about the i-process. It described something like this,’” Herwig said.
Over the next five years, evidence of stars with i-process signatures piled up. But theorists like Herwig couldn’t say where the in-between process occurs, or the exact sequence of steps by which it proceeds.
To fully understand the i-process, they needed to know the ratios of the different elements it creates. Those yields depend on how easily the relevant isotopes can capture neutrons. And to pin down the neutron capture rates, the scientists needed to study the isotopes in action at labs like FRIB. (Experiments have also taken place at Argonne National Laboratory in Illinois and other facilities.)

Supernova 1987A (center), the closest observed supernova in 400 years, arose from the core collapse of a massive star. The explosion ejected the star’s outer layers, sprinkling the surrounding space with elements. Studies of this supernova confirmed theories about the synthesis of elements up to iron.
NASA, ESA, Robert Kirshner (CfA, Moore Foundation), Max Mutchler (STScI), Roberto Avila (STScI)
Herwig discussed the mysteries of the i-process and the prospective experiments with Spyrou when he visited the Michigan State lab in 2017.
“I was hooked,” Spyrou said. “I said, ‘Just tell me which isotopes matter.’”
A Radioactive Recipe
Theorists like Herwig and experimentalists like Spyrou are now in a years-long give-and-take, where the theorists decide which isotope sequences have the largest bearing on the i-process’s final chemical cocktail, then the experimentalists fire up the accelerator to study those raw ingredients. The resulting data then helps theorists create better models of the i-process, and the cycle begins again.
In the basement of FRIB sits a particle accelerator the length of about one and a half football fields, comprised of a string of 46 sage green super-cooled containers arranged in the shape of a paper clip.
Each experiment starts with an ordinary, stable element — usually calcium. It’s fired through the accelerator at a target such as beryllium, where it splinters into unstable isotopes during a process called fragmentation. Not every nucleus will shatter exactly how researchers want it to.
“It’s like if you had a porcelain plate with a picture of an Italian city,” said Hendrik Schatz, a nuclear astrophysicist at FRIB. If you wanted a piece with just one house on it, you’d have to break a lot of plates before you got the right picture. “We’re shattering a trillion plates per second.”
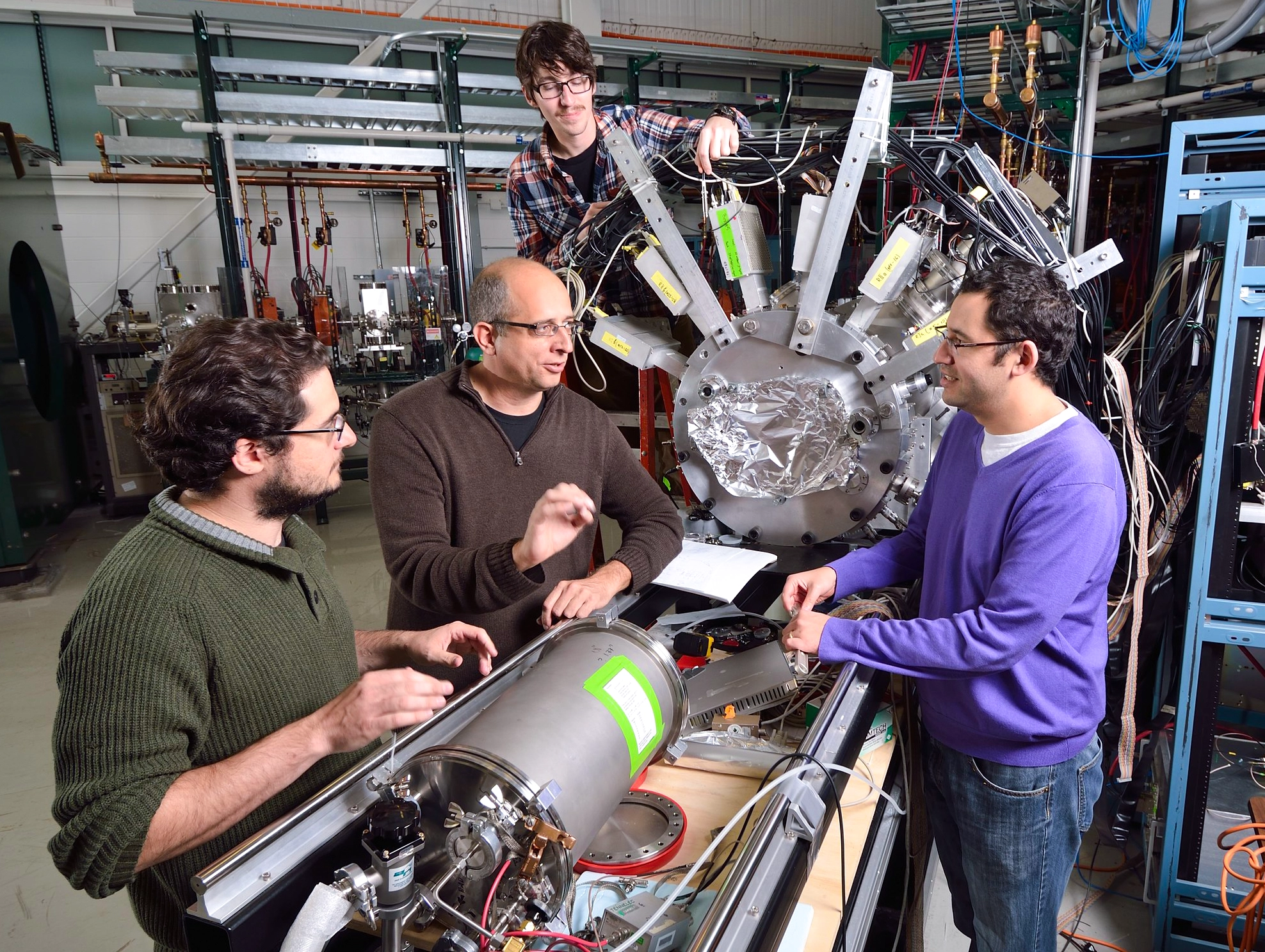
From left: Antonios Kontos, Hendrik Schatz, Zach Meisel and Fernando Montes gather around the instruments at FRIB that are used to restage the same nuclear reactions that occur inside stars.
Courtesy of Facility for Rare Isotope Beams
The shards flow through a network of pipes into a fragment separator that sorts them into isotopes of interest. These eventually end up at the SuN, a cylindrical detector 16 inches wide. With metal spokes extending out in all directions, “it kind of looks like the sun, which is fun,” said Ellie Ronning, an MSU graduate student.
Just as the nuclei enter, they begin decaying, shedding electrons and emitting flashes of gamma rays that researchers can use to decode the steps of the i-process. “No one’s been able to see these particular processes before,” said Sean Liddick, a FRIB nuclear chemist.
By measuring gamma-ray production, the researchers infer the rate at which the relevant isotopes capture neutrons (how readily barium-139 gains a neutron and becomes barium-140, to name one important example). Theorists then input this reaction rate into a simulation of the i-process, which predicts how abundant different heavy elements will be in the final chemical mixture. Finally, they can compare that ratio to the elements observed in different stars.
So far, the results seem to draw a circle right where Spyrou and her colleagues had hoped: The relative abundances of lanthanum, barium and europium match what was seen in those carbon-enhanced, metal-poor stars that so puzzled astrophysicists in the early 2000s. “We went from having these huge uncertainties to seeing the i-process fit right where we have the observations,” she said.
The i-process, however, would have taken place in the dying stars that came before those metal-poor ones and provided them with material. Right now, the data is compatible with both white dwarfs and red giants as the setting of the i-process. To see which candidate will prevail, if not both, Spyrou will need to study the neutron capture rates of more isotopes. Meanwhile, to distinguish between those candidate stars, Herwig will create better three-dimensional models of the plasma swimming inside them.
Going for Gold
For 60 years, astronomers have theorized that gold, silver and platinum all spawn during the r-process, but the exact birthplaces of these elements remain one of astrochemistry’s most long-standing questions. That’s because “r-process experiments are basically nonexistent,” Cowan said. It’s hard to reproduce the conditions of a neutron-star collision on Earth.
A 2017 observation found traces of gold and other r-process elements in the debris of a neutron-star collision, lending strong support to that origin story. But a tantalizing discovery reported this past April links the r-process to a colossal flare from a highly magnetic star.
After sorting out the i-process, the researchers in Michigan plan to apply the same tactics to the r-process. Its isotopes are even tricker to isolate; if fragmentation during the i-process is like capturing a picture of a house from a shattered plate, then the r-process means picking out only the window. Still, Spyrou is optimistic that her team will soon try out the rarer flavors of isotopes required for the express recipe, which cooks up heavy nuclei in seconds. “With the r-process, we’re close to accessing the nuclei that matter,” she said.
“But with the i-process, we can access them today,” she said. Spyrou estimates that her lab will nail down all the important i-process reactions and rates within five to 10 years. “Ten years ago,” she added, “I didn’t even know the i-process existed.”
This story was supported in part by the Council for the Advancement of Science Writing and The Brinson Foundation.