Mark Belan/Quanta Magazine
Introduction
There’s a simple story of the greenhouse effect: A blanket of carbon dioxide envelops the planet, letting sunlight in but trapping its heat. As a result, Earth warms.
But how does this actually work? Carbon dioxide amounts to only a tiny smattering of gas molecules — 0.042%, or roughly 420 parts per million — in our thick atmosphere. And yet, we know that doubling carbon dioxide levels can change the character of life on Earth.
The answer is quantum mechanics, which determines whether a molecule can interact with the right type of radiation.
Part 1: Maintaining Energy Balance
But first, we need a basic understanding of how radiation, such as sunlight, interacts with objects, such as planets.
Everything in the universe radiates, pumping out heat. A light bulb radiates heat; so does a rock sitting on the ground. Same with your phone, your body and Earth itself.
The radiation given off by an object takes the form of light, or electromagnetic waves. These magnetic and electric fields undulate as they move through space, carrying energy with them.
Hotter objects give off more heat; their waves are more energetic, oscillating with a shorter wavelength. Objects on Earth tend to be cool (generally under 30 degrees Celsius) and radiate light with relatively long wavelengths, known as infrared radiation. The sun is much hotter, about 5,000 degrees Celsius, so it radiates visible radiation with shorter wavelengths.
A radiating object will cool off unless there’s a source of heat replenishing it. For example, Earth releases heat, but it doesn’t cool down. That’s because all the heat that it loses gets replenished by the sun’s radiation. As long as Earth absorbs the same amount of heat from the sun as the amount it gives off, it will stay the same temperature — in equilibrium.
Imagine Earth with no atmosphere. Its surface butts up right against the cold vacuum of space, with no barrier in between. Even if the Earth’s surface was extremely cold, about minus 18 degrees Celsius, it would be warm enough to radiate all the heat it’s taking in. At that low temperature, the Earth and sun would already be in a happy equilibrium.
Now add an atmosphere — a thicket of gas molecules bound to the Earth by its gravitational pull. Say some of these molecules are greenhouse gases that interact with the outgoing radiation. Some of Earth’s radiation is now redirected back to its surface. Instantly, the amount of heat escaping the planet drops. But the same amount of heat is entering from the sun as before. We are out of equilibrium.
With more heat entering than leaving, the planet’s temperature begins to rise. But remember, the hotter an object, the more it radiates. So as Earth warms up, it begins pumping out more heat. This trend continues until the same amount of heat is escaping as is entering. Balance is restored at this new, hotter equilibrium.
Now, we can answer the deeper question: What makes a molecule trap heat in the atmosphere?
Part 2: The Quantum States
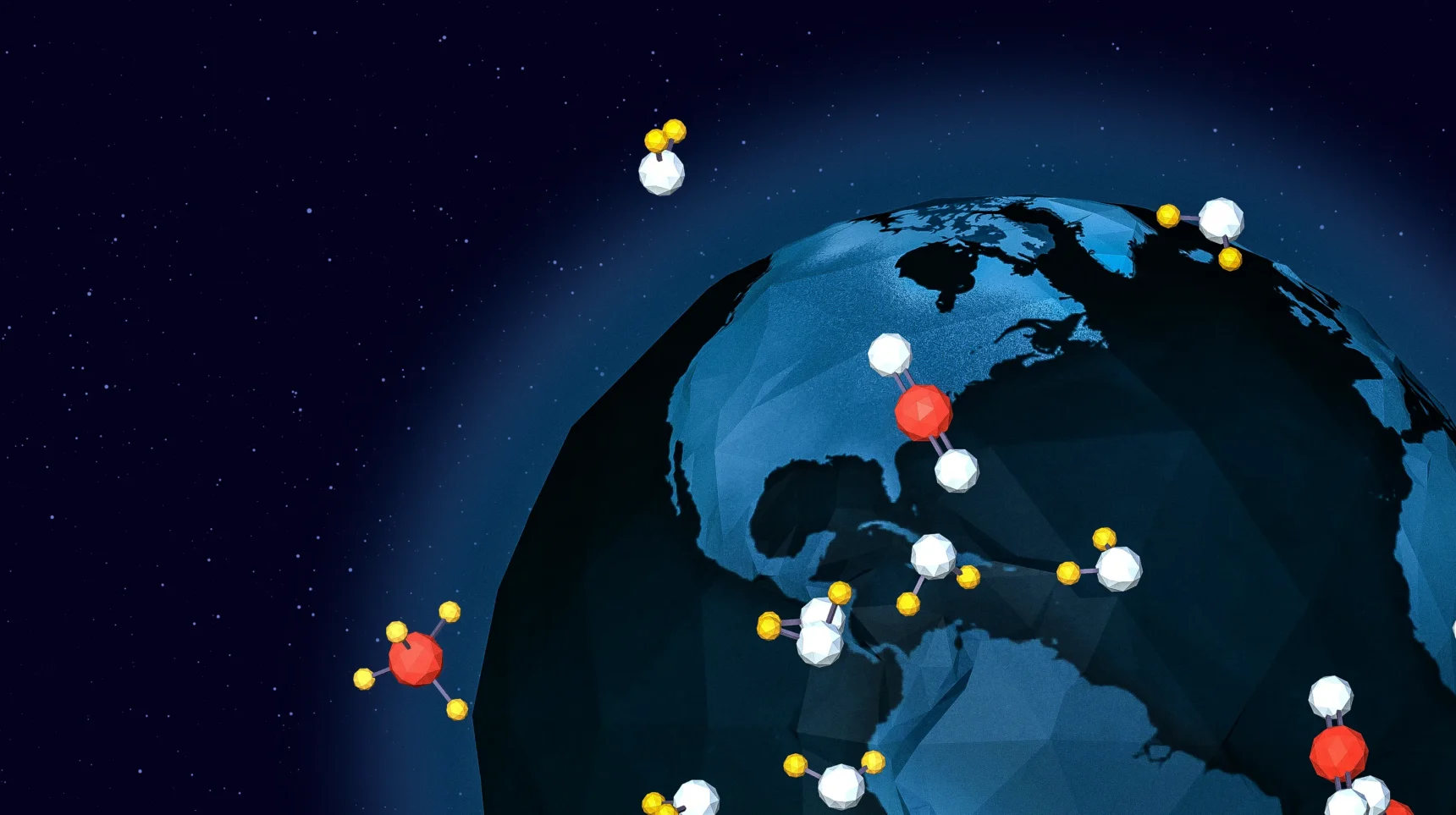
To interact with Earth’s radiation, a molecule’s electric charge needs to be off balance.
Take carbon monoxide: one carbon atom bonded to one oxygen atom. Oxygen attracts electrons more strongly than carbon does; as a result, the molecule’s electrons tend to skew towards the oxygen side, making the duo permanently off balance.
On the other hand, diatomic nitrogen, or N2, which makes up 78% of our atmosphere, is totally balanced.
Earth’s radiation produces electric and magnetic fields in the atmosphere that change over time. These shifting fields can cause an unbalanced molecule to dance. When the electric field is positive (when the wave in the figure below rises above a central horizontal axis), positive charges are pushed upward and negative charges are pulled downward. When it’s negative, the reverse happens.
An unbalanced molecule, such as carbon monoxide, acts like a positive and negative charge bonded together. The electric field makes the molecule stretch and contract. This motion — called “vibration” — requires energy. And the molecule absorbs that energy from the passing wave.
But an unbalanced molecule can’t absorb energy from just any radiation. The passing wave needs to have exactly the right wavelength to match one of the quantum states of the specific molecule. Otherwise, the wave will sail right by. (This is also why the sun’s visible light doesn’t interact with unbalanced molecules: It’s the wrong wavelength.) Because molecules have multiple quantum states, they can absorb multiple wavelengths of light. Each wavelength generates a different excited state.
The excited state lasts a few seconds at most. Then the radiation is released, beaming in a random direction. That could be along its initial trajectory toward space, or one back down toward Earth, or anywhere in between.
The molecule can also rotate around its axis. Rotations are similarly “quantized”: The light’s wavelength has to line up precisely with the molecule’s quantum state, which is determined by the molecule’s structure. To absorb energy from radiation, the wavelength has to align exactly with any of these states’ energies.
This is the definition of a greenhouse gas: any atmospheric molecule whose quantum states precisely match the wavelengths of Earth’s radiation.
Part 3: The Cast of Molecules
Most of the atmosphere is nitrogen. Nitrogen and other balanced molecules, including diatomic oxygen (O2) and lone noble gas atoms, together make up more than 99.5% of the air. None of them interact with Earth’s radiation; they are not greenhouse gases.
When it comes to the climate, the tiny remaining sliver of atmospheric molecules is where the action is.
Carbon monoxide is the simplest unbalanced molecule in air, but it makes up only 0.00001% and isn’t a big player in the greenhouse game.
Water vapor is the most important greenhouse gas; without it, the planet’s surface would be only a few degrees above freezing. Like other greenhouse gases, it is an imbalanced molecule: Its electrons prefer to congregate around the oxygen atom.
Most of Earth’s radiation isn’t energetic enough to excite water molecules into a vibrational quantum states. But it can set water spinning around the rotational excited state of one of its three axes.
Ozone and nitrous oxide also have an intrinsic charge imbalance: Earth’s radiation aligns with their quantum states that mix rotation and vibration. They are important greenhouse gases as well.
The most famous greenhouse gas is different from the rest. Carbon dioxide is not intrinsically imbalanced. Usually, its electrons are spread evenly along its length.
But its shape allows for special vibrational states: Carbon dioxide can bend, creating a temporary imbalance.
That imbalance lines up precisely with one of the wavelengths of Earth’s radiation. Carbon dioxide can interact with light by simultaneously bending and rotating. The bending temporarily creates the requisite imbalance, enabling the radiation to force it into rotation.
This uncanny coincidence — that the Earth’s radiation lines up with the energies of carbon dioxide’s mixed vibrational/rotational quantum state — is how this tiny trace of molecules, mere flecks in the air, completely dominates our climate.
Methane is nearly symmetric, too: Its four hydrogen atoms form a pyramid with the carbon atom at the center. But it can similarly bend into a temporarily unbalanced shape and rotate. Some of these rotations match up with Earth’s radiation.
Each of these gases has its own unique quirks, but they all lead to the same outcome: absorbing and redirecting some of Earth’s outgoing infrared radiation.
Part 4: Back to Earth’s Energy Balance
A redirected packet of radiation is usually reabsorbed by a nearby molecule. That means that any single packet of radiation can escape to space only after a long, random trudge through the atmosphere.
When more greenhouse gases join the party, that walk becomes longer. Even less energy escapes.
That longer walk means that the amount of heat escaping Earth declines, just as it did when the planet first gained an atmosphere. Equilibrium is again disrupted: More energy is entering than can leave. With a denser morass of radiation changing hands, the atmosphere begins to warm, all the way down to Earth’s surface.