Genes Have Harnessed Physics to Help Grow Living Things
Sip a glass of wine, and you will notice liquid continuously weeping down the wetted side of the glass. In 1855, James Thomson, brother of Lord Kelvin, explained in the Philosophical Magazine that these wine “tears” or “legs” result from the difference in surface tension between alcohol and water. “This fact affords an explanation of several very curious motions,” Thomson wrote. Little did he realize that the same effect, later named the Marangoni effect, might also shape how embryos develop.
In March, a group of biophysicists in France reported that the Marangoni effect is responsible for the pivotal moment when a homogeneous blob of cells elongates and develops a head-and-tail axis — the first defining features of the organism it will become.
The finding is part of a trend that defies the norm in biology. Typically, biologists try to characterize growth, development and other biological processes as the result of chemical cues triggered by genetic instructions. But that picture has often seemed incomplete. Researchers now increasingly appreciate the role of mechanical forces in biology: forces that push and pull tissues in response to their material properties, steering growth and development in ways that genes cannot.
Modern imaging and measurement techniques have opened scientists’ eyes to these forces by flooding the field with data that invites mechanical interpretations. “What has changed over the past decades is really the possibility to watch what happens live, and to see the mechanics in terms of cell movement, cell rearrangement, tissue growth,” said Pierre-François Lenne of Aix Marseille University, one of the researchers behind the recent study.
The shift toward mechanical explanations has revived interest in pre-genetic models of biology. For example, in 1917 the Scottish biologist, mathematician and classics scholar D’Arcy Thompson published On Growth and Form, which highlighted similarities between the shapes found among living organisms and those that emerge in nonliving matter. Thompson wrote the book as an antidote to what he thought was an excessive tendency to explain everything in terms of Darwinian natural selection. His thesis — that physics, too, shapes us — is coming back into vogue.
“The hypothesis is that physics and mechanics can help us understand the biology at the tissue scale,” said Alexandre Kabla, a physicist and engineer at the University of Cambridge.
The task now is to understand the interplay of causes, where genes and physics somehow act hand in hand to sculpt organisms.
Grow With the Flow
Mechanical models of embryo and tissue growth are not new, but biologists long lacked ways of testing these ideas. Just seeing embryos is difficult; they are small and diffusive, bouncing light in all directions like frosted glass. But new microscopy and image analysis techniques have opened a clearer window on development.
Lenne and his co-workers applied some of the new techniques to observe the motion of cells inside mouse gastruloids: bundles of stem cells that, as they grow, mimic the early stages of embryo growth.

Sham Tlili (left), Pierre-François Lenne (right), and their Aix Marseille University colleagues Simon Gsell and Matthias Merkel have unraveled a Marangoni-like flow pattern that occurs in the early stages of embryogenesis.
Courtesy of Pierre Francois Lenne
Their observations revealed that cells flow up the sides of the gastruloid, then form a stream of tissue flowing down the middle. For Lenne, the system brought to mind a droplet, and on reviewing the literature on the surface tension in a moving droplet, he hit upon the Marangoni effect.
James Thomson’s 1855 description of the Marangoni effect explained how, when two liquids that have different surface tensions meet, the fluid with the higher surface tension will pull on the other. This happens because surface tension is just the tendency of the outermost molecules in a fluid to be drawn inward by neighboring molecules. When two fluids meet, the higher-tension fluid will have a stronger pull, so the lower-tension fluid will move in the higher-tension fluid’s direction. In a wineglass, the alcohol on the wetted sides of the glass evaporates quickly, leaving a more watery liquid behind. Water has a higher surface tension than alcohol, so the watery sides drag the wine in the glass up to the top of the wetted area. It eventually drips down under its own weight, forming “tears.”
This flow of the wine up the sides and down again is similar to the flow of the tissue in the gastruloid. Indeed, when the team tested a model of Marangoni-type gastruloid tissue flow, they found what they considered a striking fit with their experimental data.
Mark Belan/Quanta Magazine; Source: Pierre-François Lenne
The Marangoni flow is a mechanical effect, but genes are involved too: They set up the surface tension difference. At first, genes produce a higher concentration of two particular proteins in one part of the blob of cells. These proteins lead to lower surface tension, and so tissue flows away from that region. The tissue moves around the periphery of the gastruloid before recirculating down its center — just as wine tears drip back down the side of a glass. The process elongates the gastruloid. It’s “a very nice example of how mechanics, coupled with all the intrinsic complexity of molecular and cellular biology, has a very important role in shaping organisms,” Kabla said.
Scales of a Feather
In 2017, Alan Rodrigues and Amy Shyer couldn’t find what they were looking for. The pair, co-leaders of Rockefeller University’s Laboratory of Morphogenesis, had been trying to figure out how the regular spacing of a bird’s feathers comes about. The popular theory at the time was that bird embryos secrete special molecules called morphogens across their skin tissues. These morphogens would then prompt genes to produce proteins at the right places to form follicles. But the researchers couldn’t find any genetic signal that would start the process.
They came to suspect that mechanical and tensile forces were playing a significant role. In a 2023 report in Science, their team found that morphogens were indeed secreted just before a feather follicle started to bud. But the morphogens didn’t seem to be influencing development on the level of individual cells. Instead, they were influencing larger swaths of tissue. The morphogens affected the tissue’s material properties, setting the stage for mechanical forces to push and pull on the tissue for follicle patterning.
“What’s really amazed us is that you might be able to get by with a relatively simple amount of instruction from the genetic and molecular level,” said Rodrigues. “Because you have additional emergent processes and properties happening at other levels.”
For Rodrigues, the big issue is how the various processes work together across length scales, from genes to cells to tissues. It’s not that everything starts on the smallest scales and builds from there. In the case of avian feather follicle development, changes at the molecular and tissue levels emerge together. The work “challenges the general view across much of biology,” Rodrigues said, “that regulation or causation emerges at the molecular level and then feeds upward across scales to dictate high-level properties such as form.”
Springing Into Action
Some proteins do affect material properties within individual cells, setting the stage for mechanical forces to act at that level too. For instance, during the embryogenesis of a fruit fly, cells in the embryo don’t just rearrange themselves; Kabla and his co-authors discovered that the cells also stretch. This stretching appears to be directly attributable to gene activity that results in a curious characteristic of the cells’ stretchiness.
Take a spring or an elastic material like a rubber band, and the material will extend in proportion to the force applied. This relationship is known as Hooke’s law, and it holds quite generally. Unless, that is, the object being stretched is in some kind of viscous fluid, in which case the amount of extension also depends on time. (Think of stirring molasses: It’s hard to stir fast.)
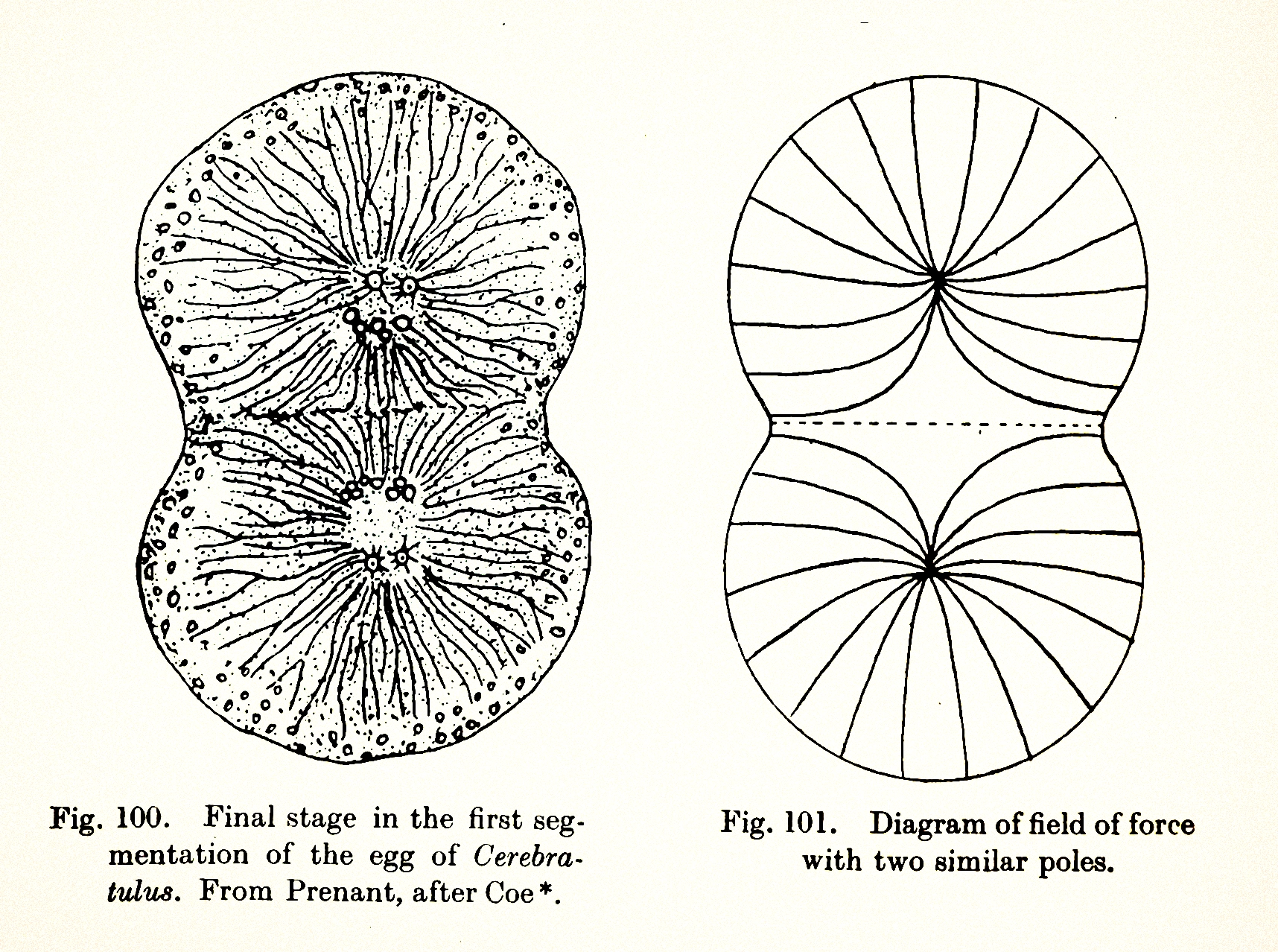
Figures from D’Arcy Thompson’s 1917 tome On Growth and Form.
Public Domain
Biological organisms appear to share this dependence on time. Several groups have measured the stretching of certain cells in the fruit fly embryo and found that their extension depends on the square root of the amount of time the force is applied. The question then becomes: Where does this behavior come from?
In a paper in Physical Review Letters in June, Konstantin Doubrovinski and colleagues at the University of Texas Southwestern Medical Center explain it in terms of the production of actin, one of the most abundant proteins in these cells. They suggest that the actin filaments effectively pull on the cell like springs as they are produced, creating resistance to the force that stretches the cells and giving rise to the observed behavior.
Doubrovinski and his team verified the role of actin by repeating the experiment using drugs that prevent the actin protein from assembling. “Essentially, the elastic response more or less completely goes away,” he said.
Kabla says that while the study makes a strong case, discussion of the stretching behavior continues. One of the challenges facing biology, he points out, is figuring out what is causing what, and whether a given phenomenon is a key driver of change, a contributing factor, or an unimportant consequence.
These questions echo similar debates over the biological significance of the geometric similarities D’Arcy Thompson cataloged more than 100 years ago. But Thompson’s central argument that these geometric forms result from underlying physical forces is standing up to modern scrutiny.
“To many of us,” Kabla said, “it seems natural that where there’s motion, mechanics is likely to be involved.”