What Can a Cell Remember?
New research investigates whether single cells can learn and remember.
Kristina Armitage/Quanta Magazine
In 1983, the octogenarian geneticist Barbara McClintock stood at the lectern of the Karolinska Institute in Stockholm. She was famously publicity averse — nearly a hermit — but it’s customary for people to speak when they’re awarded a Nobel Prize, so she delivered a halting account of the experiments that had led to her discovery, in the early 1950s, of how DNA sequences can relocate across the genome. Near the end of the speech, blinking through wire-framed glasses, she changed the subject, asking: “What does a cell know of itself?”
McClintock had a reputation for eccentricity. Still, her question seemed more likely to come from a philosopher than a plant geneticist. She went on to describe lab experiments in which she had seen plant cells respond in a “thoughtful manner.” Faced with unexpected stress, they seemed to adjust in ways that were “beyond our present ability to fathom.” What does a cell know of itself? It would be the work of future biologists, she said, to find out.
Forty years later, McClintock’s question hasn’t lost its potency. Some of those future biologists are now hard at work unpacking what “knowing” might mean for a single cell, as they hunt for signs of basic cognitive phenomena — like the ability to remember and learn — in unicellular creatures and nonneural human cells alike. Science has long taken the view that a multicellular nervous system is a prerequisite for such abilities, but new research is revealing that single cells, too, keep a record of their experiences for what appear to be adaptive purposes.

Barbara McClintock
Science History Images
In a provocative study published in Nature Communications late last year, the neuroscientist Nikolay Kukushkin and his mentor Thomas J. Carew at New York University showed that human kidney cells growing in a dish can “remember” patterns of chemical signals when they’re presented at regularly spaced intervals — a memory phenomenon common to all animals, but unseen outside the nervous system until now. Kukushkin is part of a small but enthusiastic cohort of researchers studying “aneural,” or brainless, forms of memory. What does a cell know of itself? So far, their research suggests that the answer to McClintock’s question might be: much more than you think.
Brainless Learning
The prevailing wisdom in neuroscience has long been that memory and learning are consequences of “synaptic plasticity” in the brain. The connections between clusters of neurons simultaneously active during an experience strengthen into networks that remain active even after the experience has passed, perpetuating it as a memory. This phenomenon, expressed by the adage “Neurons that fire together, wire together,” has shaped our understanding of memory for the better part of a century. But if solitary nonneural cells can also remember and learn, then networks of neurons can’t be the whole story.
From an evolutionary perspective, it makes sense for cells outside a nervous system to be changed by their experiences in ways that encourage survival. “Memory is something that’s useful to all living systems, including systems that predated the emergence of the brain by hundreds of millions of years,” said Sam Gershman, a cognitive scientist at Harvard University.
Acellular slime molds, foraging for food, lay down chemical traces that remind them where they’ve been. Bacteria compare present and previous conditions as they move through chemical gradients towards more favorable environments. Gershman has a hunch that these “more ancient form[s] of memory” may play an important, complementary role to synaptic plasticity — so much so that he recently added a wet lab to his operation to systematically study the single-celled ciliate Stentor coeruleus.
Ciliates might sound like an unconventional focus for a cognitive scientist, but the study of memory in these unicellular creatures dates back to the early 20th century. The zoologist Herbert Spencer Jennings, in his Behavior of the Lower Organisms, detailed experiments with a similar ciliate, S. roeselii, as early as 1906. Found in freshwater ponds around the world, these trumpet-shaped cells anchor themselves to their environment with a sticky “holdfast” and draw in floating food particles by beating hairlike cilia.
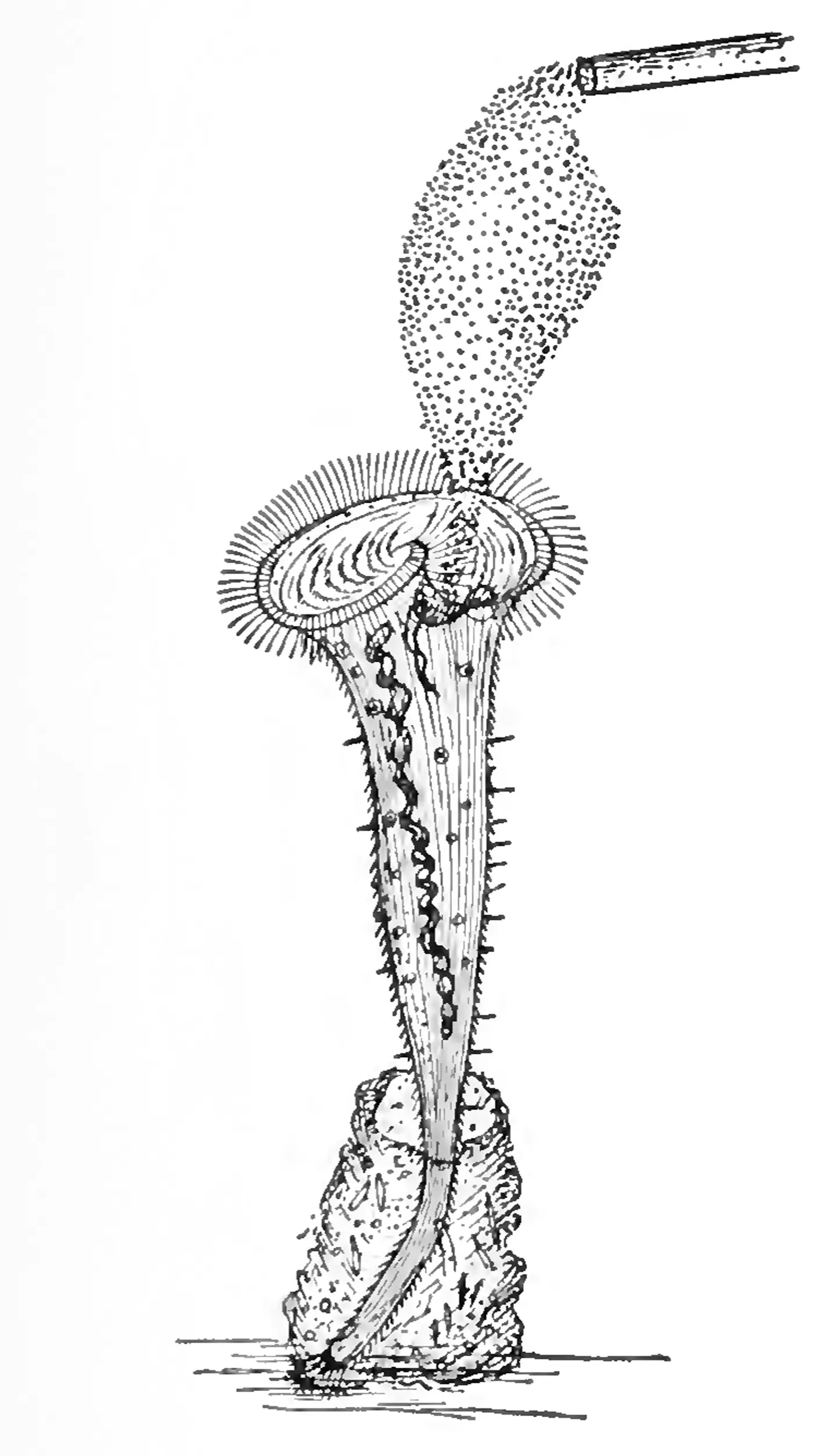

In 1906, experiments by Herbert Spencer Jennings (right) suggested that the single-celled ciliate Stentor roeselii (left) can learn — that it was “changed by the experiences it has passed through,” he wrote.
Herbert Spencer Jennings via Archive (left); American Philosophical Society/Science Source
In a series of experiments, Jennings repeatedly squirted an irritating red dye at some of these ciliated protists sourced from a nearby pond and observed how the organisms reacted. First, he found, individuals bent away from the glass pipette with which he dispensed the dye. If that didn’t prevent the irritation, the protists used their cilia to spit water at the pipette. And if that still failed to clear the dye, they would contract sharply back into their holdfasts. Bend, spit, hide. Having established this sequence of responses, Jennings decided to test S. roeselii’s memory by repeating the experiment after a brief delay.
When the ciliates emerged from the holdfast after about half a minute in hiding, they once again encountered the dye. Would S. roeselii go through the whole avoidance sequence again, Jennings wondered, or would the organism instead be “changed by the experiences it has passed through”? In other words, would the cell learn? The answer, he found, was “specially interesting.” Faced again with the relentless dye, it contracted right away, skipping the preamble. After a final sequence of emergences and contractions, the ciliate eventually got fed up, pulled up stakes and swam away, presumably looking for a less noxious place to settle down.
The dominant view of unicellular behavior in Jennings’ era was that organisms like S. roeselii were driven by “tropisms,” automatic responses to external factors such as light, chemical gradients and gravity. But Jennings’ work showed that a unicellular organism could ratchet up its response over a short period of time, which indicated that it factored previous experience into its actions — that it could, in effect, remember.
Ironically, Stentor memory was nearly forgotten. Jennings’ experiments were widely considered not to be reproducible; for the next century, research into cellular learning would be routinely dismissed and even considered fringe science. Then, in 2010, Gershman’s colleague Jeremy Gunawardena took an interest in it. Gunawardena, a mathematician turned systems biologist who was until very recently at Harvard Medical School, went digging in the library stacks and discovered that the only real attempt to reproduce Jennings’ experiments had been made in the late 1960s on Stentor coeruleus, a completely different organism. Buoyed by this obvious error, he managed to convince a graduate student and one of his postdocs to join him in a years-long, nights-and-weekends skunkworks project to reproduce Jennings’ work with the correct organism. When their results were published in Current Biology in 2019, Jennings was vindicated: S. roeselii can, in fact, “change its mind.”
Gunawardena and Gershman run a discussion group for their peers in the field of cellular learning. “It’s not that large of a universe of people working on this,” Gershman said. They’re also history buffs; their papers often feature tender portraits of the maligned scientists this work is now redeeming. Having rehabilitated Jennings, they’ve moved on to the lesser-known Beatrice Gelber, an iconoclastic scientist who left her post at the University of Chicago in the 1960s after she claimed to have “trained” a different single-celled ciliate, a paramecium, to associate an uncoated metal wire with food — think Pavlov’s petri dish. Gelber’s rigorous work is some of the only research on associative learning in single cells. As with Jennings, Gunawardena believes, she was dismissed in her day for largely ideological reasons; her trained paramecia refuted a prevailing orthodoxy that cells couldn’t learn.
Now, he says, we know better.
A Cell’s Perspective
If an intracellular mechanism for memory exists in brainless, unicellular organisms, then it’s possible we inherited some form of it, given the advantages it presents. All eukaryotic cells, including our own, trace their evolutionary origins to a free-living ancestor. That legacy echoes in our every cell, yoking our fates to the vast unicellular realm, where creatures such as protozoans navigate threats, seek succor and sense their way from life to death.
Most of us have a hard time stepping outside our subjective, introspective experience to imagine what memory might look like for such a cell. But for Nikolay Kukushkin, who trained as a molecular biologist, it’s an easy leap. “Really, when I close my eyes, I’m inside the cell,” he said on a video call from his lab at the Center for Neural Science at NYU.
A cell’s entire existence, Kukushkin explained, takes place in the warm darkness of a multicellular body. From that perspective, what we might call “experience” is patterns of chemicals spaced in time: nutrients, salts, hormones and signaling molecules from neighboring cells. These chemicals affect the cell in different ways — sparking molecular or epigenetic changes, for example — and at different rates. All of this affects how the cell responds to new signals. At the level of the cell, that’s what memory is, Kukushkin believes: an embodied response to change. There’s no distinction between memory, the memorizer and the act of remembering. “For the cell,” he said, “it’s all the same.”
To make this idea clear, Kukushkin recently decided to try and locate, in a cell, a feature of memory common to all animals, first described by the German psychologist Hermann Ebbinghaus in 1885. Ebbinghaus was his own guinea pig: He spent years memorizing and re-memorizing lists of nonsense syllables to measure his recall. He found that it was easier to remember sequences of syllables when he paced his memorization sessions, rather than studying everything at once — a “spacing effect” that should be familiar to anyone who’s ever crammed for a test and realized they should have started studying earlier.
Since Ebbinghaus identified the spacing effect, it has “proven to be one of the most unshakeable properties of memory in many different animals,” Kukushkin wrote in a recent essay. It’s such such a widespread phenomenon — found in disparate life forms as humans, bees, sea slugs and fruit flies — that Kukushkin wondered if it might reach all the way down to the cell. To find out, he’d need to measure just how responsive nonneural cells are to spaced chemical patterns.
Kukushkin and his colleagues began by growing human kidney cells and immature nerve cells in isolation. Then they tried to mimic what they knew about neurons’ chemical “experience.” Their key innovation was a way to measure these cells’ internal responses to chemical cues by using a DNA sequence that’s part of many cell-signaling pathways, including those used by neurons, called the cAMP response element, or CRE. For the experiment, this gene was a proxy for memory. By engineering both the cell lines to produce a glowing protein whenever CRE was activated, they were able to measure when the cells formed a memory, and how long that memory persisted.
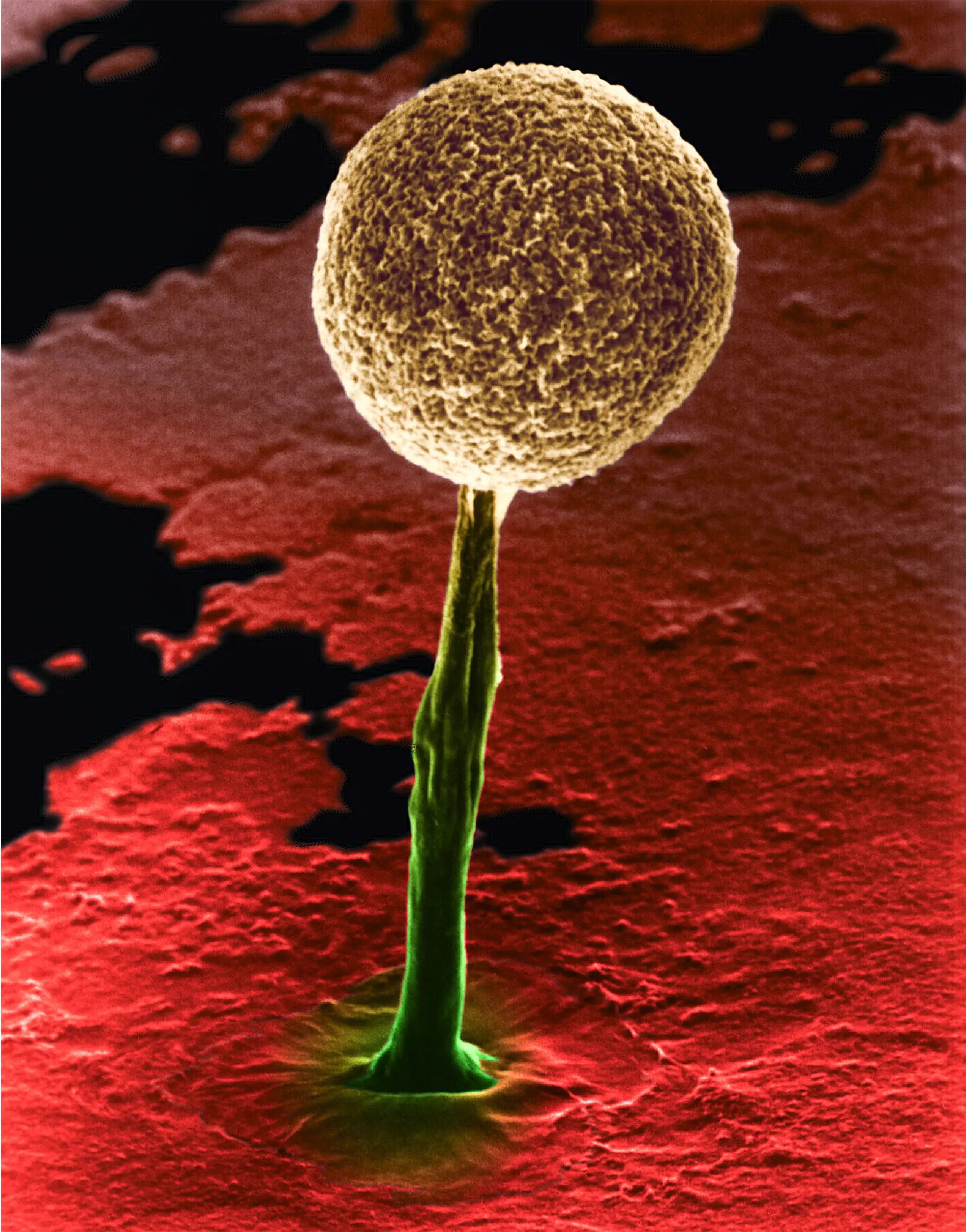

Research in diverse organisms — such as acellular slime molds (left), which lay down chemical reminders, and planarian worms (right), which respond to training after being decapitated — suggests that nonneural cells record their experiences.
Dennis Kunkel Microscopy/ Science Source; Bazzano Photography
Then, in a process Kukushkin described as a tedious choreography of clockwork pipetting, they exposed the cells to precisely timed bursts of chemicals that imitated bursts of neurotransmitters in the brain. Kukushkin’s team found that the both the nerve and kidney cells could finely differentiate these patterns. A steady three-minute burst activated CRE, making the cells glow for a few hours. But the same amount of chemicals, delivered as four shorter pulses spaced 10 minutes apart, lit up the petri dish for over a day, indicating a lasting imprint — a memory.
Kukushkin’s findings suggest that nonneural cells can count and detect patterns. Even though they can’t do it at the speed of a neuron, they do remember, and they appear to remember a stimulus for longer when it is delivered at spaced intervals — a hallmark of memory formation in all animals.
Intuitively, this makes sense, Gershman said. From the perspective of the cell, or any other living system that shows the spacing effect, spaced information is evidence of a fairly consistent, slow-moving environment: a steady world. Massed information, on the other hand — a singular burst of chemicals or an all-night cram session — might represent a fluky event in a more chaotic environment. “If the world is changing really fast, you should forget things [more easily], because the things that you learned are going to have a shorter shelf life,” Gershman said. “They’re not going to be as useful later on, because the world will have changed.” These dynamics are as relevant to a cell’s existence as they are to ours.
Kukushkin, who has recently taken to calling himself a “molecular philosopher,” is fairly certain his findings would have been the same regardless of the type of cell he used. “I’m accepting bets for anybody’s favorite cell line showing a spacing effect,” he said. “I think it should be the default assumption that memory is a continuous process — that all these single cells memorize, that plants memorize, that neurons and all kinds of cell types memorize in the same way. The burden of proof shouldn’t be in proving that it’s the same. The burden of proof should be in proving that it’s different.”
Gershman agrees. “In a brain, the dynamics [of memory] concern neurons signaling to each other: a multicellular phenomenon,” he said. “But in a single cell, maybe we’re talking about the dynamics within a cell of molecules at different timescales. Different physical mechanisms can give rise to a common cognitive process, similar to how I could use a pen or a pencil or typewriter or computer to write a letter.”
At the end of the day, the letter — that is, the memory — is what matters.
Structural Bias
Part of the reason that science has been hesitant to embrace cellular-scale memory is sociological, Gunawardena said. The findings of early researchers such as Jennings and Gelber were memory-holed because they didn’t resonate with the prevailing theories of their time: Jennings’ discovery of memory in Stentor went against the dogma of “tropisms,” which inspired the behaviorist psychology dominant in Gelber’s day. Both of these views presumed a living world populated by biological automata, cycling through preprogrammed responses. Cells that can learn and adapt didn’t figure into such models.
“We all have our ideologies,” said Gunawardena, who is now at Pompeu Fabra University in Barcelona. “It’s just a natural part of how humans deal with the world. … In science, we’ve really underplayed how important those biases can be in organizing scientific communities, and in setting what is considered appropriate and not appropriate science.”

“I think it should be the default assumption that memory is a continuous process,” said Nikolay Kukushkin, a neuroscientist at New York University — that “all kinds of cell types memorize in the same way.”
Arina Voronova
It’s also an issue of semantics. Like all important terminology, “memory” is loaded, imprecise and defined variously by different disciplines. It means one thing to a computer scientist and another to a biologist — to say nothing of the rest of us. “When you ask a normal person what memory is, they think of it introspectively,” Kukushkin said. “They think, ‘Well, I close my eyes and I think back to yesterday, and that’s memory.’ But that’s not what we’re studying in science.”
In neuroscience, Kukushkin writes, the most common definition of memory is that it’s what remains after experience to change future behavior. This is a behavioral definition; the only way to measure it is to observe that future behavior. Think of S. roeselii snapping back into its holdfast, or a lab rat freezing up at the sight of an electrified maze it’s tangled with before. In these cases, how an organism reacts is a clue that prior experience left a lingering trace.
But is a memory only a memory when it’s associated with an external behavior? “It seems like an arbitrary thing to decide,” Kukushkin said. “I understand why it was historically decided to be that, because [behavior] is the thing you can measure easily when you’re working with an animal. I think what happened is that behavior started as something that you could measure, and then it ended up being the definition of memory.”
Behavior tells us that a memory has formed but says nothing about why, how or where. Further, it’s constrained by scale. Take Aplysia californica, a muscular sea slug with enormous neurons (the largest is about the size of a letter on a U.S. penny). Neuroscientists love to conduct memory experiments on Aplysia because its physical responses are easy to measure — poke it and it flinches — and they map cleanly to the handful of sensory and motor neurons involved.
The sea slug, Kukushkin said, can complicate neuroscience’s behavioral bias. Say you shock its tail, triggering a defensive reflex. If you shock it again the next day and find that the defensive reflex is stronger than it was before, that’s behavioral evidence that the slug remembers its initial shock. Any neuroscientist would associate it with a memory.

The California sea hare (Aplysia californica) has enormous neurons, making it easy to study as a model for memory.
Gerald Robert Fischer
But what if (apologies to the squeamish) you take that sea slug apart and leave only its immobile neurons? Unlike the intact creature, the neurons can’t retract, so there will be no visible response. Is the memory gone? Certainly not, but without external validation, a behavioral definition of memory breaks down. “We no longer call that a memory,” Kukushkin said. “We call that a mechanism for a memory, we call that synaptic change underlying memory, we call that an analogue of memory. But we don’t call that a memory, and I think that it’s arbitrary.”
Perhaps a definition of memory should extend beyond behavior to encompass more records of the past. A vaccination is a kind of memory. So is a scar, a child, a book. “If you make a footprint, it’s a memory,” Gershman said. An interpretation of memory as a physical event — as a mark made on the world, or on the self — would encompass the biochemical changes that occur within a cell. “Biological systems have evolved to harness those physical processes that retain information and use them for their own purposes,” Gershman said.
So, what does a cell know of itself? Perhaps a better version of Barbara McClintock’s question is: What can a cell remember? When it comes to survival, what a cell knows of itself isn’t as important as what it knows of the world: how it incorporates information about its experiences to determine when to bend, when to battle and when to make a break for it.
A cell preserves the information that preserves its existence. And in a sense, so do we. As today’s cellular memory researchers revisit abandoned experimental threads from the past, they too are discovering what memory owes to its context, how science’s sociological environment can determine which ideas are preserved and which are forgotten. It’s almost as though a field is waking up from a 50-year spell of amnesia. Fortunately, the memories are flooding back.