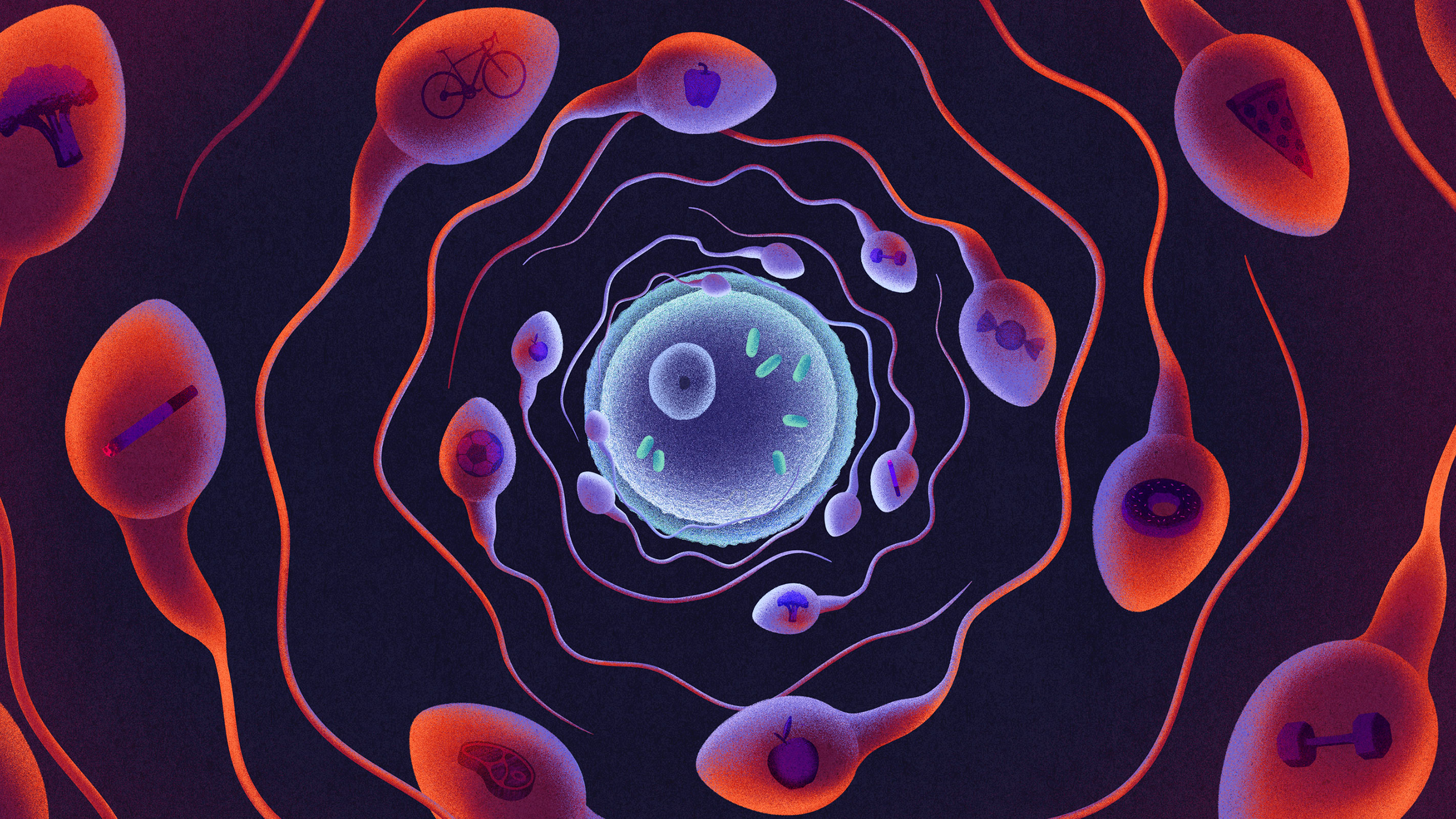
How Dad’s Fitness May Be Packaged and Passed Down in Sperm RNA
A father’s fitness, as measured by diet and exercise, is encoded by sperm RNA and epigenetically affects his offspring, according to research in mice.
Myriam Wares for Quanta Magazine
Introduction
The standard sperm-meets-egg story posits that sperm cells are hardly more than bundles of shrink-wrapped DNA with tails. Their mission is simple: Deliver a father’s genes into a mother’s egg for sexual reproduction. Just about all other aspects of a developing embryo, including its cellular and environmental components, have nothing to do with dad. Those all come from mom.
But nearly two decades of studies from multiple independent labs threaten to rewrite that story. They suggest that dad’s gametes shuttle more than DNA: Within a sperm’s minuscule head are stowaway molecules, which enter the egg and convey information about the father’s fitness, such as diet, exercise habits and stress levels, to his offspring. These non-DNA transfers may influence genomic activity that boots up during and after fertilization, exerting some control over the embryo’s development and influencing the adult they will become.
The findings, so far largely described in mouse models, could end up changing the way we think about heredity. They suggest “that what we do in this life affects the next generation,” said Qi Chen, a reproductive and developmental biologist at the University of Utah Medical School who is among the pioneers of this research. In other words: What a father eats, drinks, inhales, is stressed by or otherwise experiences in the weeks and months before he conceives a child might be encoded in molecules, packaged into his sperm cells and transmitted to his future kid. The researchers have largely zeroed in on RNA molecules, those short-lived copies of DNA that reflect genetic activity at a given time.
It’s a tantalizing notion. But the mechanistic details — how experience is encoded, how it’s transferred from sperm to egg, and whether and how it affects a developing embryo — are not easy to unpack, especially given the challenges of conducting research in human subjects. For this reason, and because of the potentially textbook-rewriting implications of the findings, researchers, including those spearheading the work, are cautious about overselling their results.

Colin Conine has shown that RNAs packaged in sperm alter gene expression in mouse embryos, pointing to a pathway by which a dad’s choices can epigenetically affect his offspring.
Courtesy of Colin Conine
“It’s still very hand-wavy,” said the epigeneticist Colin Conine of the University of Pennsylvania Perelman School of Medicine and Children’s Hospital of Philadelphia, who has been trying to uncover the mechanics of how sperm RNA can contribute nongenetic information to progeny. Some elements of the story are clear, he said: Researchers have significant evidence that the environment can regulate sperm RNAs, that these molecules transmit traits to offspring and that they can regulate embryonic development after fertilization. “We just don’t have really any understanding of how RNAs can do this, and that’s the hand-wavy part,” Conine said.
But evidence keeps piling up. Most recently, in November 2025, a comprehensive paper published in Cell Metabolism traced the downstream molecular effects of a father mouse’s exercise regimen on sperm microRNAs that target genes “critical for mitochondrial function and metabolic control” in a developing embryo. The researchers found many of those same RNAs overexpressed in the sperm of well-exercised human men.
“This study shows that paternal exercise can confer benefits — enhanced endurance and metabolic health — to offspring,” said Chen, who was not involved in the study. “It’s a powerful reminder that many sperm-mediated epigenetic effects are deeply adaptive in nature.”
The possibility that a previously undocumented avenue of inheritance is at play is too important to ignore. That’s why the researchers are now hunkering down in their labs to trace out the molecular processes that would have to operate for a father’s here-and-now experience to be transferred as developmental instructions to his partner’s egg.
Epigenetic Avenues
In most animals, a sperm cell is tiny compared to an egg cell. In humans, an egg contains 10 million times the volume of a sperm and contributes most cellular components — nutrition, cytoplasm, mitochondria and other organelles, the molecular machinery to make proteins, and more — to a zygote (a newly fertilized egg that hasn’t started dividing). Plus, a mother provides the environment within which an embryo and then fetus develops and grows. As a result, the effect of a mother’s health on her children has long been scrutinized, including at the molecular level. But over the past 15 years or so, the evidence for some kind of non-DNA inheritance of paternal experience has also been strengthening.
“There are many different labs that have done diet and stress studies, and typically the readouts of those in the next generation are either metabolism or behavioral changes,” Conine said. Feed a male mouse a high-fat or low-protein diet, or take him away from his mom when he is young, and his offspring will inherit traits, such as changes in mitochondrial function, related to those environmental conditions. These traits aren’t necessarily detrimental. For instance, mouse fathers exposed to nicotine sire male pups with livers that are good at disarming not just nicotine but cocaine and other toxins as well.
There is a survival logic here, said Oliver Rando, an epigeneticist at the University of Massachusetts Chan Medical School who led the nicotine study. It’s reasonable to expect that offspring will experience an environmental context similar to that of their parents. Biologically priming them for those conditions could therefore help them survive.

The epigeneticist Oliver Rando suspects that sperm RNAs control early gene regulation in an embryo. If he’s right, he will have helped discover a new fact of life, he said.
Nick Rhind
“You might think of this as a way to tell your kids something useful for them so that they can be better at dealing with the world they inherit,” said Rando, a father of two boys.
But logic does not make the story true. That’s why Chen, Rando, Conine and other researchers have been working to uncover the mechanistic components of dad-based epigenetic heredity to add to what is already known about these processes in mothers. “Epigenetics” refers to heredity processes that do not change the DNA sequences of genes, but rather involve when and to what degree genes are expressed and made into functional proteins. Epigeneticists focus on the molecular biology that unfolds around genomic and chromosomal frameworks that can switch genes on and off in response to internal and external cues.
This kind of differential gene expression is central to some of biology’s greatest wonders — for example, the fact that all cells in the human body have the same DNA, and yet brain cells differ dramatically from liver, skin and blood cells. Some cues that trigger changes in gene expression are programmed into DNA, while others come from the environment — for example, a dearth of calories or nutrients due to food deprivation, or rising cortisol levels due to stressors such as the absence of parental care. These conditions affect the kinds of metabolites and other molecules circulating in our bodies, which influences what kinds of reactions and genomic processes cells can carry out.
Some molecules involved in epigenetic processes, such as methyl or acetyl groups, interface directly with DNA or bind to proteins attached to DNA. These actions loosen up or batten down portions of the genome and are akin to opening or closing doors to specific genes.
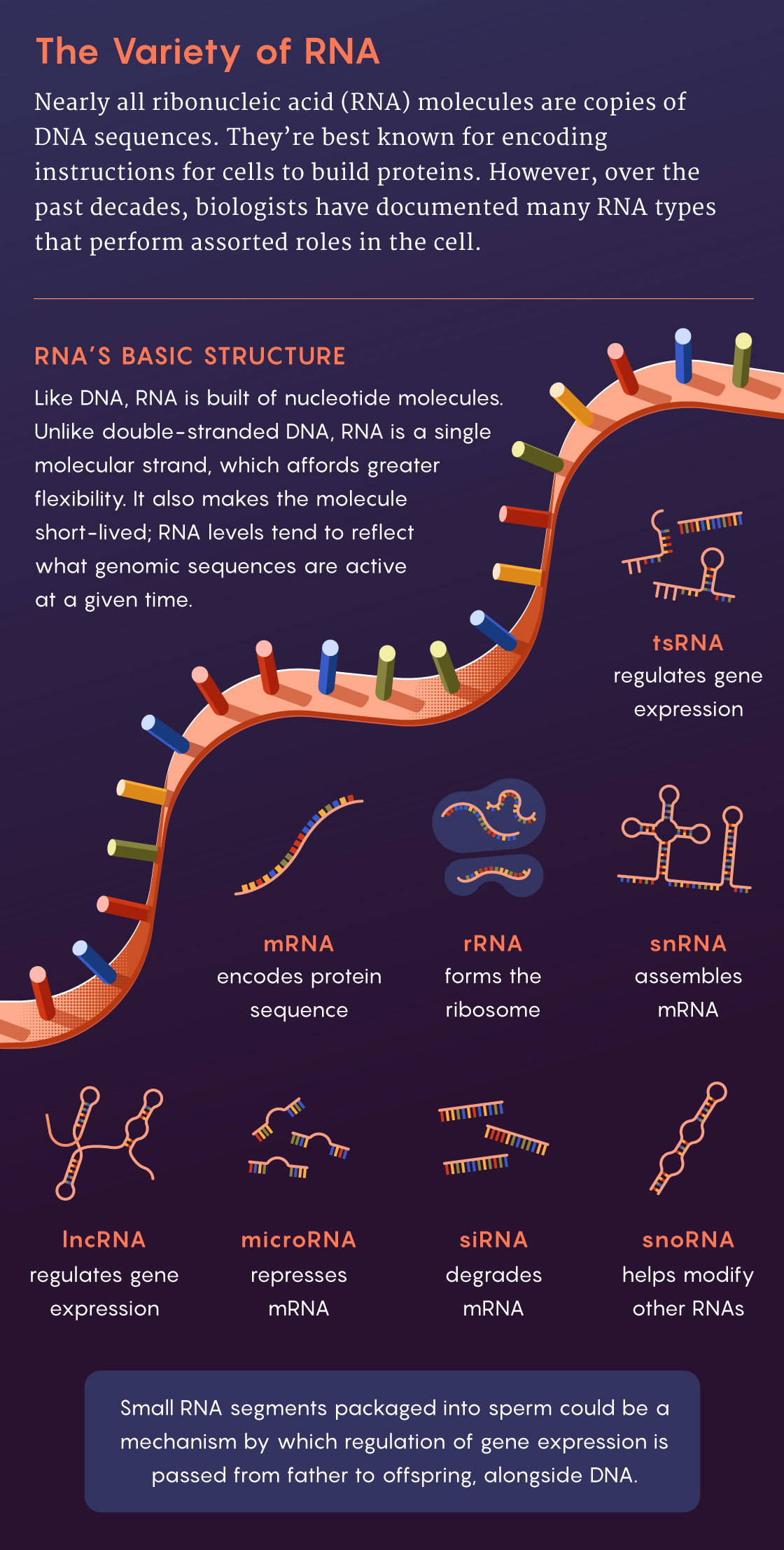
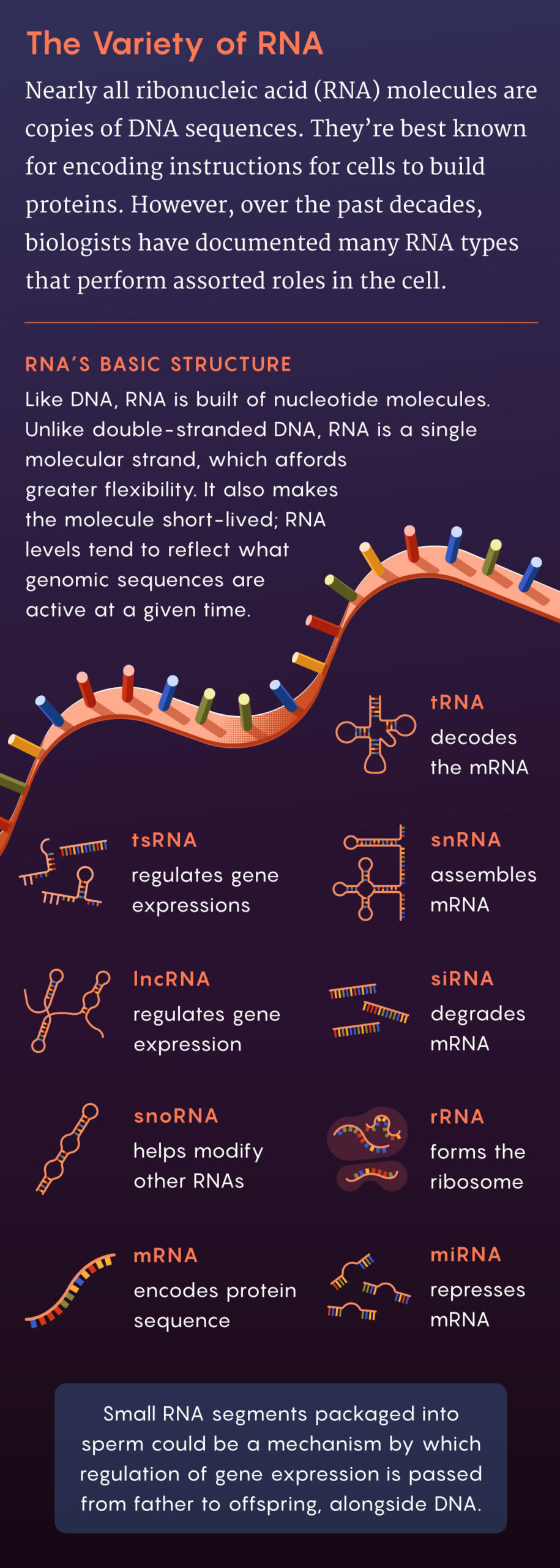
RNA molecules — flexible, ephemeral versions of DNA sequences — can also intervene in gene expression. But because they are relatively short-lived, sometimes surviving mere minutes or hours before degrading, they have been overlooked as epigenetic regulators. Since the 1990s, their roles have been clarified, as has their longevity: Certain RNAs can survive for weeks or longer. Some RNAs (such as long noncoding RNAs, or lncRNAs) regulate gene expression by modifying DNA or its proteins. Others, known as microRNAs, alter or repress other RNAs, including those that would otherwise be translated into proteins; this discovery was awarded the 2024 Nobel Prize in Physiology or Medicine.
Could sperm carry RNA or other molecules that then participate in epigenetic processes in the embryo? It seemed plausible to some researchers, but it would take a whole lot of experimental work to put the pieces together.
A Package of RNA
There are three core questions that biologists need to resolve to confirm that sperm cells transmit epigenetic inheritance. The first is how a father’s body physically encodes lived experience, such as stress, diet, exercise or nicotine use, in the form of molecules — for example, as RNAs circulating in blood that reflect gene expression in tissues. The next centers on how molecularly encoded experience could make its way into sperm cells. The third traces how those signals in sperm could become epigenetic vectors during and after fertilization to specify observable traits, known as phenotypes, in offspring.
In a series of studies beginning in 2012, Qi Chen started answering all three questions. In what he describes as one of the most serendipitous discoveries of his career, his team at the Chinese Academy of Sciences in Beijing used sequencing techniques to inventory the short RNA molecules present in mice sperm cells.

In 2012, Qi Chen found RNAs crowding into sperm heads with DNA. He’s spent his career documenting what he calls the “sperm RNA code.”
Courtesy of Qi Chen
They were shocked to see a subset of the RNAs drastically increasing in concentration as sperm cells matured, and then crowding into the sperm heads with DNA. This same class of RNAs was abundant in the blood serum of various vertebrates, ranging from fish to humans, they found. All of this pointed to the possibility that information-carrying molecules were being transferred into the reproductive cells.
It got even more interesting when Chen, who moved to the U.S. academic circuit in 2015, and his team collected sperm RNAs from male mice fed different diets. The RNA assemblages in mice reared on high-fat foods differed dramatically from those in mice that were fed normal diets. And when the researchers injected the RNAs from the sperm of the fat-eating mice into a zygote, some of the male offspring showed metabolic issues associated with a high-fat diet.
The experiments hinted at a seemingly heretical possibility, Chen said: that “certain acquired traits during paternal exposure can be ‘memorized’ in the sperm and inherited by the offspring.” After characterizing a pathway that regulates sperm RNAs, in 2019 Chen dubbed this channel of heredity the “sperm RNA code,” which he suggested “programs the metabolic health of offspring.”
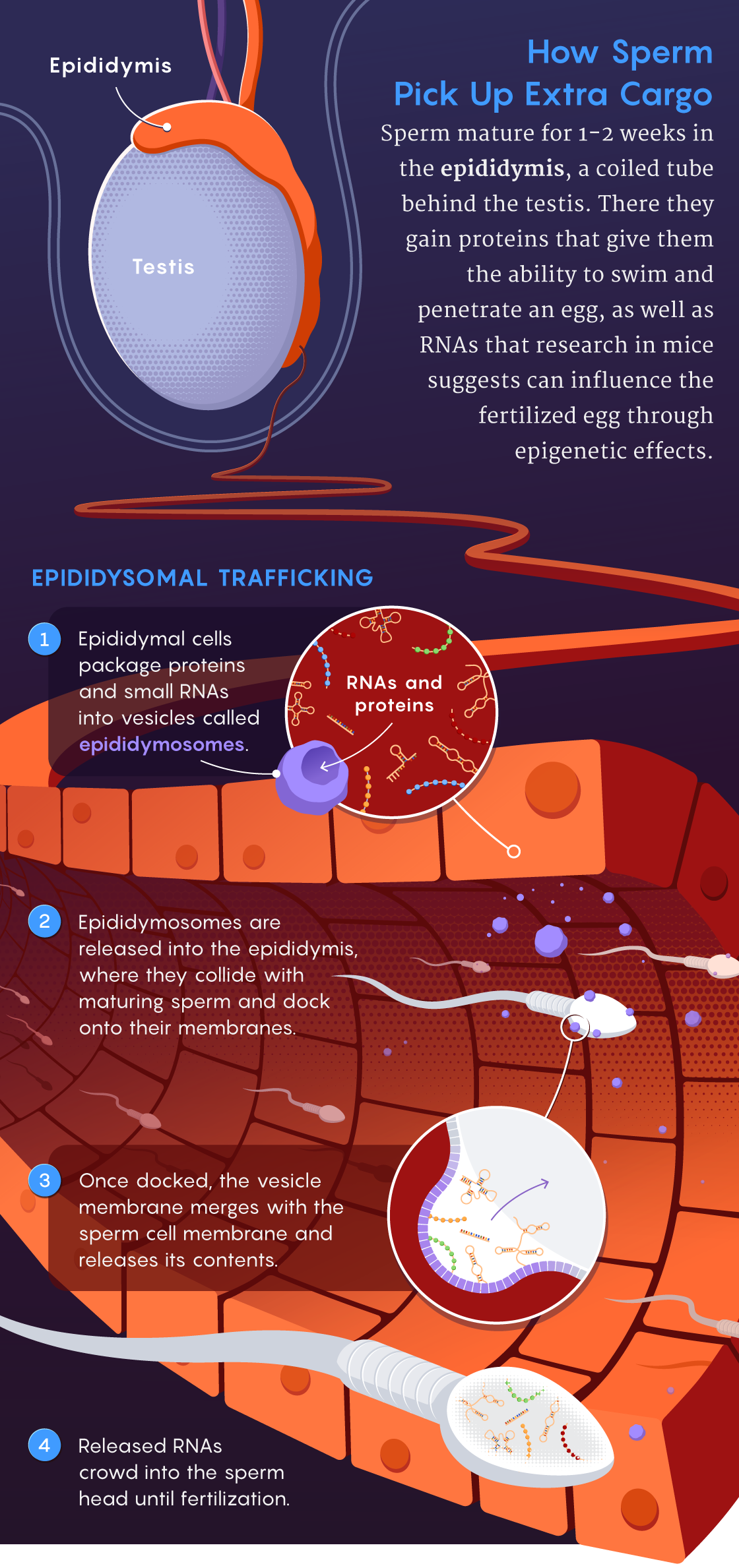
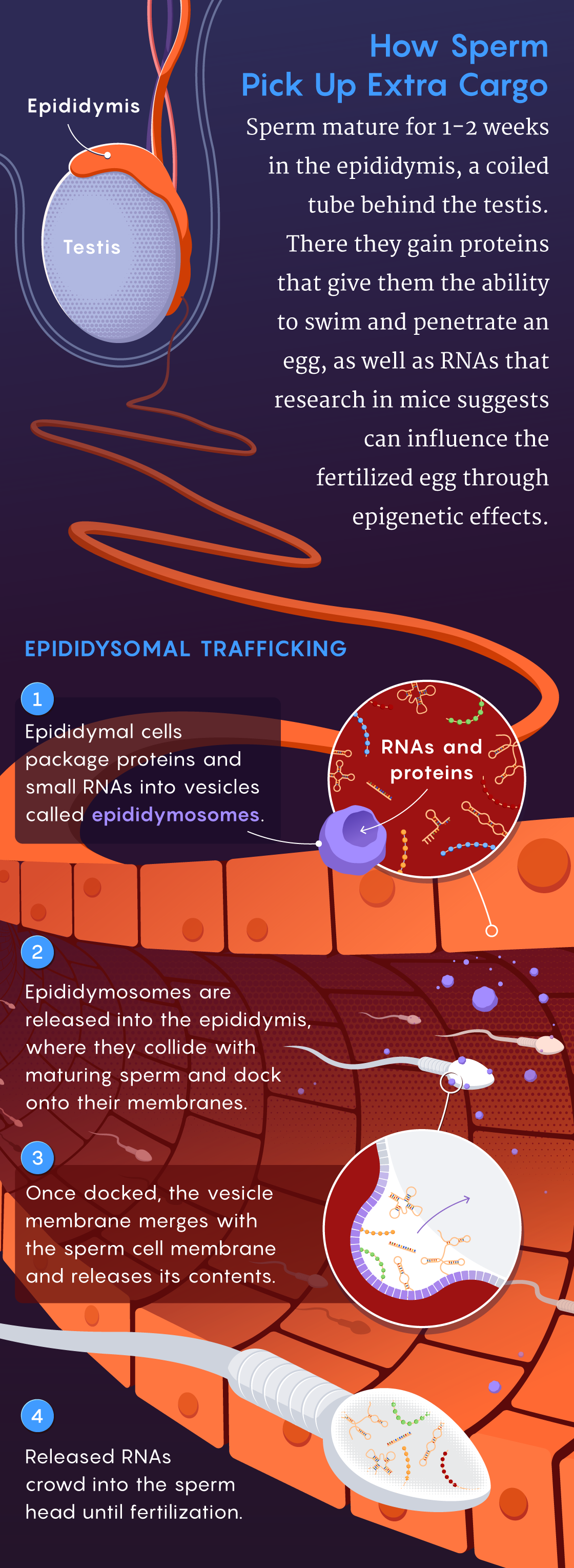
He wasn’t the only one to become fixated on this idea. Around the same time, in an article published in Developmental Cell in 2018, Rando’s team reported the use of biochemical techniques to characterize where and when RNAs are packaged into sperm cells and how those RNAs might change during this process. They were trying to answer the question: “What tissue could be responsible for choosing what to tell sperm to tell kids?” he said. One logical place to start looking was the epididymis. Within this tubular organ attached to the back of the testicle, sperm cells undergo a maturation process, taking about one to two weeks in most mammalian species, before they become ready for fertilization.
Rando’s data showed that sperm cells gain almost all their small RNAs when they are in the epididymis. Using techniques for tracking specific RNA molecules, the scientists observed RNAs getting packaged into virus-size capsules, called epididymosomes, which shuttled the molecules into sperm.
“This shows how small RNAs can get trafficked between the body’s nonreproductive cells, like those in the epididymis, and germline cells,” such as sperm, Rando said. “The epididymis has been emerging as a key location for paternal effects. Certainly the epididymis has to be taken seriously as a potential sensor of the world.”
A Molecular Snapshot
By “sensor of the world,” Rando is referring to the first stage of a presumed paternal-effects mechanism — when a male’s body translates a lived condition, such as a high-fat diet, rigorous exercise or toxin exposure, into molecular signals. The epididymis then provides a route for the second step, packaging those signals for the next generation.
This is also where investigations by Isabelle Mansuy come in. At the University of Zurich and the Swiss Federal Institute of Technology Zurich, she studies the molecular and cellular mechanisms of epigenetic inheritance in mammals.

Isabelle Mansuy has traced the ways that consequences of stress from early life trauma alter metabolic pathways through five generations of mice.
© Philippe Rossier / Ringier Media Switzerland
In one line of her research, she focuses on processes that transmit the molecular effects of traumatic stress to subsequent generations by focusing on extracellular vesicles (EVs) that circulate in blood. Shed by almost every type of cell in the body, including in the epididymis (the epididymosomes are EVs), they carry a diversity of molecular cargo, such as RNAs, proteins, lipids and metabolites. Because EVs circulate in blood just about anywhere in the body and can cross cell membranes, they provide a potential means to transfer molecules and the biological cues they carry between bodily tissues and reproductive cells. Notably, RNAs tend to survive longer when packaged in EVs.
Mansuy creates trauma in mice by subjecting the animals to conditions such as restraint or maternal separation when they are young. Then she searches for molecular changes in reproductive cells that could cause similar consequences of trauma to manifest in the children or even grandchildren of the animals who directly endure it.
She’s shown that traumatic stress alters metabolic pathways, especially those that involve lipids, in exposed male mice and their offspring. She has also found a similar metabolic profile in humans who experienced high stress in childhood. In mice, some of the metabolic changes remained discernible through five generations — a rare data-backed finding for epigenetic inheritance cascading through generations.
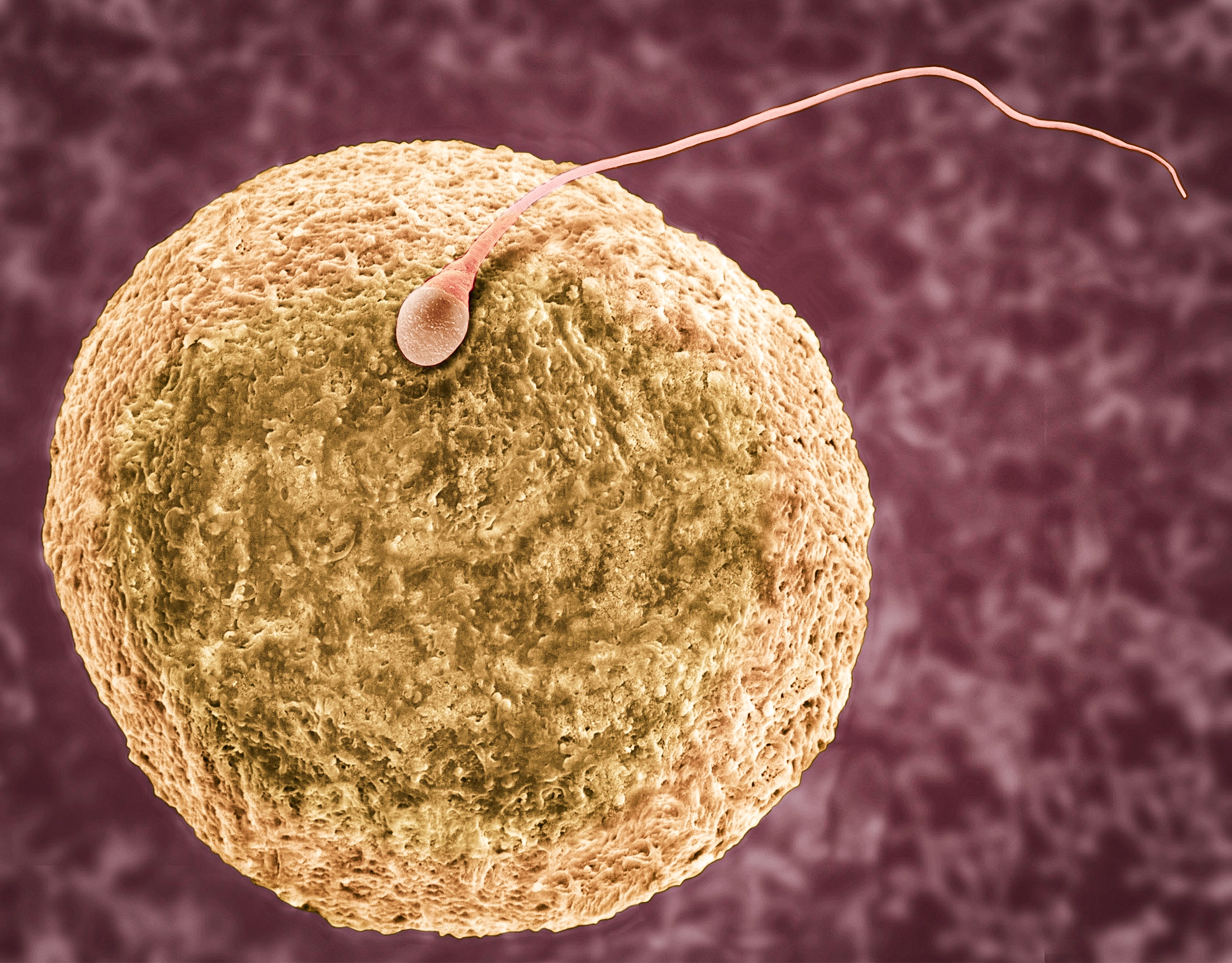
An egg cell is enormous compared to a sperm cell and contributes nearly all cellular components to a growing embryo — except the sperm’s half of the genome, and maybe embryonic and epigenetic regulators, according to recent research.
Dennis Kunkel Microscopy/Science Source
In March 2025, in a preprint uploaded to biorxiv.org, Mansuy and colleagues reported that EVs in mice can transport certain RNAs, metabolites and lipids linked to early-life stress from circulating blood to sperm, with consequences for offspring. The offspring produced by these sperm cells had stress-related metabolic dysfunction as adults and bore the stress signatures in their own sperm RNA. “These changes imply a mechanistic link between sperm RNA modifications and phenotypic features in the offspring,” Mansuy’s team concluded in their paper, which has not yet been peer-reviewed.
Phenotypic Translation
Perhaps the trickiest step to understand is how sperm-borne molecules could influence an adult’s observable traits. In one form of experiment, researchers extract all the sperm RNA from mice that have been raised under stressful or health-altering conditions. Those isolated RNAs are then injected into a zygote. Pups that emerge usually “get the dad’s phenotypes,” Conine said, suggesting that the RNAs alone confer traits from dad to offspring.
But how? During early development, epigenetic processes reign. As one fertilized cell divides into two, and those cells divide again, and so on, one set of DNA instructions is dynamically and repeatedly reprogrammed. The growing body specializes into different cell types and is sculpted into a sequence of increasingly complex forms. It’s possible, then, that early epigenetic alterations to the genome could have significant downstream effects on an adult.
A team of researchers, including Xin Jin (top) and Xi Chen of Nanjing University, traced the epigenetic effects of paternal exercise in mice.
Nanjing University
Research out of Conine’s lab, published in 2024, showed that sperm microRNAs alter gene expression in mouse embryos. Experiments like these, he said, support the idea that offspring can inherit paternal traits via the transfer of non-DNA molecular stowaways in sperm.
The recent Cell Metabolism paper took this idea a step further by tracing a mechanism by which this can happen. A team of more than two dozen Chinese researchers focused on the epigenetic transmission of exercise benefits, homing in on a set of microRNAs that reprogram gene expression in the early embryo. These changes ultimately result in skeletal muscle adaptations in adult offspring that enhance exercise endurance. The researchers found that well-exercised mice had more of these microRNAs in their sperm than sedentary mice did. When these microRNAs were transferred into zygotes, the adults they grew into were more physically fit, with more mitochondria in skeletal muscle and higher endurance.
But how did the molecules generate the exercise-positive phenotype? In experiments, the researchers found that the microRNAs suppressed a particular protein, which had the effect of boosting genes related to mitochondrial activity and metabolism.
Intriguingly, the sperm of physically trained male humans also hosted higher levels of many of the same microRNAs than those of untrained cohorts. “This cross-species conservation suggests a potential role for these sperm mi[cro]RNAs in intergenerational exercise adaptations in humans,” the researchers wrote.
The First Draft
The notion that a father’s lived experience can become recorded by his body, transmitted to his gametes and relayed to his offspring is no longer as outlandish as it once seemed. Many researchers in the field are willing to float speculative visions of what could be going on, even as they acknowledge that gaps remain.
“Our hypothesis is that the epididymis ‘sees’ the world and alters the small RNAs it produces in response,” Rando said. “These RNAs are then delivered to the zygote upon fertilization and control early gene regulation and development to shape offspring health and disease.”
Conine speculates that once certain RNAs make their way into the egg, they trigger “a cascade of changes in developmental gene expression that then leads to these phenotypes” of the father showing up in the next generation. Remarkably, this unfolds even though the sheer volume of the sperm’s contents is so much less than an egg’s contents, including the relative amounts of RNA.
The full picture of how paternal experience and behavior might epigenetically influence offspring is not nearly in hand. Researchers are currently piecing the story together, one experiment at a time, rather than proving out every step sequentially in the same set of organisms. One of the gaps is in the characterization of what RNA and perhaps other epigenetic factors do in the zygote to modify genomic activity as it unfolds during development, Mansuy said.
“We are still blind men describing for the first time different parts of the same elephant,” Chen said. “The underlying mechanism is almost certainly an orchestra of a sperm RNA code and factors beyond that.”
Confirming the findings in humans would take enormous effort, but it would be key to turning these findings in mice into “informed medical advice,” Chen said. This would require well-controlled experiments following multiple generations, tracking diet, exercise, aging and environmental exposures, while also using advanced tools to decode sperm-packaged molecules — and then looking for strong correlations between the molecular and phenotypic data.
Even amid the uncertainties, researchers are cautiously moving forward as they learn to believe the results of their own experiments. If they’re right, they will have discovered a new fact of life, Rando said. When he thinks about his two boys, he wonders what he might have done differently when he was younger, before they were born, that might have tweaked his RNA profile in ways that would affect them today.
“We don’t know enough yet to develop guidance like that,” Rando said. “Maybe we will get there.”