Mark belan/Quanta Magazine
Introduction
Life has influenced Earth’s atmosphere going back billions of years. But until two centuries ago, when humans started burning fossil fuels on an industrial scale, the most significant living climate controllers were organisms invisible to us: single-celled microbes. Small but mighty, microorganisms are nature’s chemists. At the very bottom of all biological processes, microbes break down, transform and supply the nutrients required for life and bring elements into biochemical cycles across the planet — atmosphere, ocean, earth and biosphere.
“The microbial biodiversity that we do not see with our naked eye sustains the biodiversity that we do see,” said Tom Battin, an environmental scientist who studies microbial ecology at the Federal Polytechnic School of Lausanne. “The microbes are like the conductors of the biogeochemical Earth orchestra. They make the music.”
Microbes convert inert nitrogen and phosphorus, for example, into forms life can use to build DNA molecules. They are responsible for at least 50% of global photosynthesis, which removes carbon dioxide from the atmosphere. They also return carbon dioxide to the atmosphere when breaking dead organisms down into their constituent molecules. As microbes drift into the deep sea, they bring carbon with them, storing it as sediment and then, deeper in the earth, as rock. The cells are even found in clouds, where they act as seeds around which ice crystals form.
“I wish people were aware of this invisible world that is working like mad behind the scenes,” said Lisa Y. Stein, a climate change microbiologist at the University of Alberta. “Plants, microbes, water, air — it’s all one system working in synergy.”
Microbes’ planetary effect dates back to life’s origins, when ancient cells first began emitting methane, a greenhouse gas that likely warmed Earth’s early atmosphere. Then around 2.7 billion years ago, in a major transition for the planet, chlorophyll-based photosynthesis evolved in cyanobacteria, which gained the ability to use sunlight to make sugar out of carbon dioxide and water — and released oxygen as a byproduct. Over hundreds of millions of years, microbes’ oxygen emissions filled the atmosphere, causing the extinction of most anaerobic life while creating the conditions for the emergence of land plants. Those plants went on to transform a mostly dead landscape into a paradise for large, complex life forms like us.
Throughout the history of life on Earth, microbes have acted as master regulators of our planet’s climate system. But now we have overpowered microbes’ climatological effects. On this warming planet, with greenhouse gas emissions rising at dangerous rates, microbiologists argue it’s time to better value our invisible colleagues. And as fellow regulators of Earth’s climate, we must understand how our actions affect microbes and learn how to work with them.
These short stories about scientists working at the leading edge of climate microbiology reveal the vital role microbes play in our biosphere and climate system, and illuminate new possibilities for collaborating with these incredible natural chemists.
────────
Kristina Armitage/Quanta Magazine
The Methane Eaters
A very long time ago, at the dawn of life on Earth, the earliest microbes kicked off the relationship between life and the atmosphere that contains it. Ancient archaean cells used the reaction between hydrogen gas and carbon to make energy for themselves, and released methane gas as a by-product. Because methane is a potent greenhouse gas, many scientists suspect that these first methanogens, or methane makers, warmed the planet some 3.5 billion years ago — making the place habitable for life forms that would follow.
Today, methane has become a problem for Earth’s life. Scientists estimate that this primary molecular component of natural gas, which humans burn for fuel, is responsible for nearly one-third of the global warming that has occurred since the start of the Industrial Revolution. And methane emissions are accelerating: From 2020 to 2022, they grew at the fastest rate since observations began. Interestingly, this recent rise was not driven by direct human emissions but by methanogen microbes responding to the changes humans have wrought.
“The methanogens are waking up,” Stein said. Warm conditions produce more food for these microbes, which thrive in rotting vegetation, as is found in tropical wetlands and thawing Arctic permafrost. And as the carbon cycle speeds up, methanogens have more carbon to feed on and release even more methane.

A thawing permafrost bank at Gates of the Arctic National Park in Alaska.
NPS Climate Change Response
However, in microbes, atmospheric scientists and microbiologists also see the potential to reduce methane levels and turn down the planet’s thermostat. Methane is a powerful greenhouse gas, with at least 80 times more warming power than carbon dioxide, but it stays in the atmosphere for less time. If we can decrease methane emissions today, experts say, we can potentially prevent half a degree Celsius of projected global warming by 2100.
Unfortunately for us, the microbes that eat methane, called methanotrophs, grow at a slower pace than the methanogens. Stein estimates it would take a millennium for methanotrophs’ methane eating to catch up with methanogens’ methane production. “They don’t grow overnight. Eating methane is not an easy thing to do,” she said.
To do this, methanotrophs have evolved enzymes that can grapple with the extremely strong and stable bonds between methane’s atoms. “These enzymes are phenomenal,” said Jessica Swanson, a biophysicist at the University of Utah. She studies one of these enzymes, a membrane protein called pMMO — one of only two known enzymes that can process methane at room temperature. Many methanotrophs are jam-packed with pMMO, which performs the first step of metabolizing the gas.
Swanson specializes in modeling these membrane proteins, an effort that will support other researchers looking to use pMMO to break down methane outside of a cell. Until then, we need microbes to consume methane for us, she said. She is collaborating with microbiologists to design bioreactors that efficiently grow methanotrophs so they can capture methane from the air or sources like landfills.
One challenge is that methane is present in the atmosphere at very low concentrations: For every million molecules, only two are methane. “We want to maximize the amount of methane that gets to the microbes and make the reactors as efficient as possible, to go after low-concentration emissions,” Swanson said.
Stein, meanwhile, is focused on recruiting wild methanotrophs to the cause. She’s working to design and deploy vegetated artificial islands that can be placed in lakes, marine systems or rivers to attract methanotrophs. Rather than engineering microorganisms to speed up methane consumption, Stein values working with naturally occurring microbes and ecosystems. “There are plenty of natural solutions to lengthen the timeline between now and our demise,” she said.
────────
The Rainmakers
A
t an observatory atop Puy de Dôme, a 4,800-foot inactive volcano in central France, the microbiologist Pierre Amato samples clouds. The skies, he said, are far from sterile. Every cubic meter of air contains anywhere from 10 to 10 million microbes, depending on the altitude, location, season and time of day.
The atmosphere is a hostile place for a microbe. Any cell drifting in the skies is blasted with UV rays and desiccated. “Surviving in a cloud is quite special,” Amato said. Some constituents of the aeromicrobiome form defensive spores or have pigments that absorb UV light; others are fully exposed. Some have no way to cope with the conditions and quickly die; others grow slowly while aloft, consuming and producing carbon-based molecules, which Amato measures in the lab. “They have to deal with the conditions and utilize what they can,” he said.

Instruments at the Puy de Dôme Observatory in France measure the optical, biochemical and physical properties of clouds.
BAUER Alexandre/Shutterstock
Many of these microbes are doing more than just surviving. They also seem to play an important role in clouds: They make it rain. On land, the model cloud microbe Pseudomonas syringae infects and damages plants by producing what are known as “ice-nucleation proteins” (INPs), which cause water to freeze at relatively high temperatures (just below 0 degrees Celsius). When the bacterium is emitted into the atmosphere, these INPs generate ice particles — a process called ice nucleation that is the first step in rain formation in cold clouds.
“Clouds are basically floating lakes that don’t fall because the droplets are too small,” said Cindy Morris, a plant pathologist at France’s National Research Institute for Agriculture, Food and Environment. “You need to kick off a process to aggregate the droplets so they’re big enough to fall.”
INPs seem to nudge this process along. Lab studies of the proteins suggest that their looped, helical configuration interacts with surrounding water molecules, lining them up into structures that encourage the formation of frozen droplets called ice nuclei. When conditions are right, other cold droplets stick to the frozen one. And when the drop grows large enough, it falls as precipitation.
Morris is studying whether this process might be a joint microbe-plant effort. Pseudomonas and other microbes are released into the atmosphere from plants along with water vapor. The rainfall these microbes induce, according to this theory, benefits the plants on the ground — feeding back into the so-called bioprecipitation cycle. The microbes benefit, too. INPs are structurally complex, and the microbes that expend the energy to make them are more likely to rain down from the sky. INPs therefore give Pseudomonas a survival advantage, Morris said. They may have helped INP-producing strains of the bacteria spread around the world. Indeed, Pseudomonas seem to have no biogeography; they are citizens of the world, found everywhere. Only Iceland, which is isolated from the jet stream, has unique populations of the bacteria, Morris said.
Pseudomonas are only the best-studied ice-nucleating microbes; others also make INPs and seem to seed rainfall. “Feedbacks like this are beneficial for both the vegetation and the microbe,” Amato said. The microbes get dispersed and the plants get rain.
While the localized impacts seem clear, researchers say that bioprecipitation has not been studied well enough to know its significance to the planet’s climate. “We need a model,” Amato said. He is collaborating with physicists to simulate the microbes’ movements through the atmosphere and to connect those movements to the climate. Ideally, he said, he’d be able to use weather data to back-calculate the origin of a microbe found in a cloud over Puy de Dôme. Using that data, he could uncover new links between microbes’ metabolic activity and shifts in atmospheric chemistry.
────────
The Nitrogen Wars
Plants have always lived with microbes. Before ancient plants evolved roots some 400 million years ago, microbes helped them absorb nutrients, and today they still provide support. Nitrogen is a prime example. Nitrogen gas is all around us, making up 78% of the atmosphere. All living things need the element to make biomolecules such as DNA, but most species can’t make use of the N2 molecule, nitrogen’s gaseous form. Microbes unlock inert nitrogen gas and convert it into reactive forms of nitrogen, such as nitrate and ammonia, that plants and other organisms can use.
But there are also more antagonistic relationships. Nitrifying microbes, which oxidize ammonia before plants can take it up, have long competed with plants for nitrogen. In fact, only about 50% of nitrogen applied as fertilizer is taken up by crops; the rest runs off into waterways as nitrate pollution or is eaten by microbes, some of which turn it into nitrous oxide, a potent greenhouse gas. Nitrous oxide is responsible for more than 10% of global warming to date, and between 1980 and 2020 emissions of this gas rose 40%.
To ensure their crops get enough nutrients, farmers apply more and more fertilizer. But plants can take up nitrogen only so fast. Nitrifying bacteria fill in the gap, metabolizing any excess fertilizer more quickly and releasing nitrogen pollution.

A tractor spews fertilizer onto a field. Nitrifying bacteria consume excess fertilizer not taken up by crops, releasing the greenhouse gas nitrous oxide.
Tsirika/iStock
Applying more fertilizer isn’t the answer to plants’ nitrogen deficiencies, said Christina Hazard, an environmental microbiologist at the French National Center for Scientific Research. “We need strategies to help plants take up nitrogen more efficiently.”
One approach under development is to dose the soil with molecules, made naturally by plants, that deter nitrifying microbes. However, those natural molecules dissipate quickly, and longer-lasting synthetic versions of these compounds can have a harmful effect. “The compound itself can impact the biodiversity of microorganisms in the soil,” many of which support plant health, Hazard said.
Her approach instead comes from within the microbiome. Her team has identified types of viruses that infect nitrifying microbes and slow down their activity. Crucially, these viruses are specific to their hosts, and those targeting nitrifiers shouldn’t harm other microorganisms in the soil. Hazard’s team is currently testing these viruses in the lab and will soon do so in the field. Other virus-based biotherapies are already on the market, including one that treats a citrus disease.
This kind of treatment would slow the microbes down, give plants more time to take up nitrogen, and reduce microbial production of nitrous oxide. “Fertilizers speed up the nitrogen cycle,” Hazard said. By focusing instead on managing the soil microbiome, it might be possible to improve crop yields while cutting greenhouse gas emissions.
────────

The Living Ice
T
he unworldly beauty of glaciers and the drama of calving ice sheets have become iconic images of climate change. But ice is more than frozen water.
“The ice is alive,” Battin said. Microbes such as bacteria, algae and viruses live on, inside and under glaciers. When a glacier melts, it is not just the loss of a chunk of frozen water — a geophysical effect of climate change — but also the loss of an ecosystem.
Ice supports biological communities found nowhere else on Earth. Alexandre Anesio, an Arctic microbiologist at Aarhus University in Denmark, argues that ice is a biome just like the forest or the desert. “On ice, there are microscopic plants [algae] and a whole food chain — they are just not as visible,” said Anesio, who studies the Greenland Ice Sheet, the second largest in the world.
In summer, the surface of the ice melts, filling tiny pores with water. Under the intense summer sun, algal cells bloom. Bacteria grow on the algae, viruses infect the bacteria, and fungi decompose dead material. These microbial ecosystems can support larger organisms, such as tardigrades and insect larvae.

The ice-loving alga Chlamydomonas nivalis has a red carotenoid pigment that stains snow pink or red — a phenomenon known as “watermelon snow” or “blood snow.”
David Katz/Adobe Stock
Ice microbes are specially adapted to their harsh conditions. Their enzymes must operate at much lower temperatures than other organisms’ enzymes. They’re also exposed to intense sunlight: In Greenland at the peak of summer, the sun shines almost 24 hours a day. To protect their chlorophyll from the sun’s relentless radiation, ice algae make orange and red pigments, which give some ice sheets a purplish-brown appearance. “Some of these organisms are found other places; some we only find in glaciers,” Anesio said.
When a glacier melts, its diversity of cryophilic microbes is lost. Even if global warming is limited to 1.5 degrees Celsius, a goal that looks increasingly out of reach, models predict that half of Earth’s glaciers will melt by 2100. Already, many have vanished, including every glacier in Venezuela and Slovenia, along with their microbial inhabitants.
Cryo-microbiologists are studying these ice-loving microbes in the wild while they can, and they will create a biobank to store backup records in case species go extinct. Battin plans to collect cells, permafrost soils, DNA and other samples for long-term storage. “We’re discovering this biodiversity, and at the same moment, it’s disappearing already,” he said.
────────
The Microbiome’s Microbiome
Ocean-drifting microbes called phytoplankton, including single-celled algae, cyanobacteria and dinoflagellates, make their own food out of almost nothing. These masters of photosynthesis use solar energy to cause reactions between carbon dioxide and water in specialized organelles, making oxygen and organic carbon. But phytoplankton can’t live on carbon dioxide alone. To get all the critical nutrients they need, they must barter with other microbes that float along with them.
“No organism on Earth lives in isolation,” said Shady Amin, a microbial oceanographer at New York University Abu Dhabi. But how does a cell floating in the vast open ocean keep its community close by?
Phytoplankton take advantage of fluid dynamics, which work differently at the single-cell scale, Amin said. Fish and other large creatures swim freely, move against the current, and leave behind a trail of molecules. But microscopic ocean microbes go with the flow. Whatever they excrete moves with them in a tiny chemical cloud, slowly diffusing away.
This means phytoplankton can establish a microenvironment in the water around them. They excrete food and chemicals to attract other microbes. Some produce sugary polymers called polysaccharides that other bacteria physically attach themselves to, as if they’re holding on to a rope. And some phytoplankton release compounds to inhibit the growth of unfriendly bacteria. This microbial community that surrounds a phytoplankton cell is called the phycosphere.
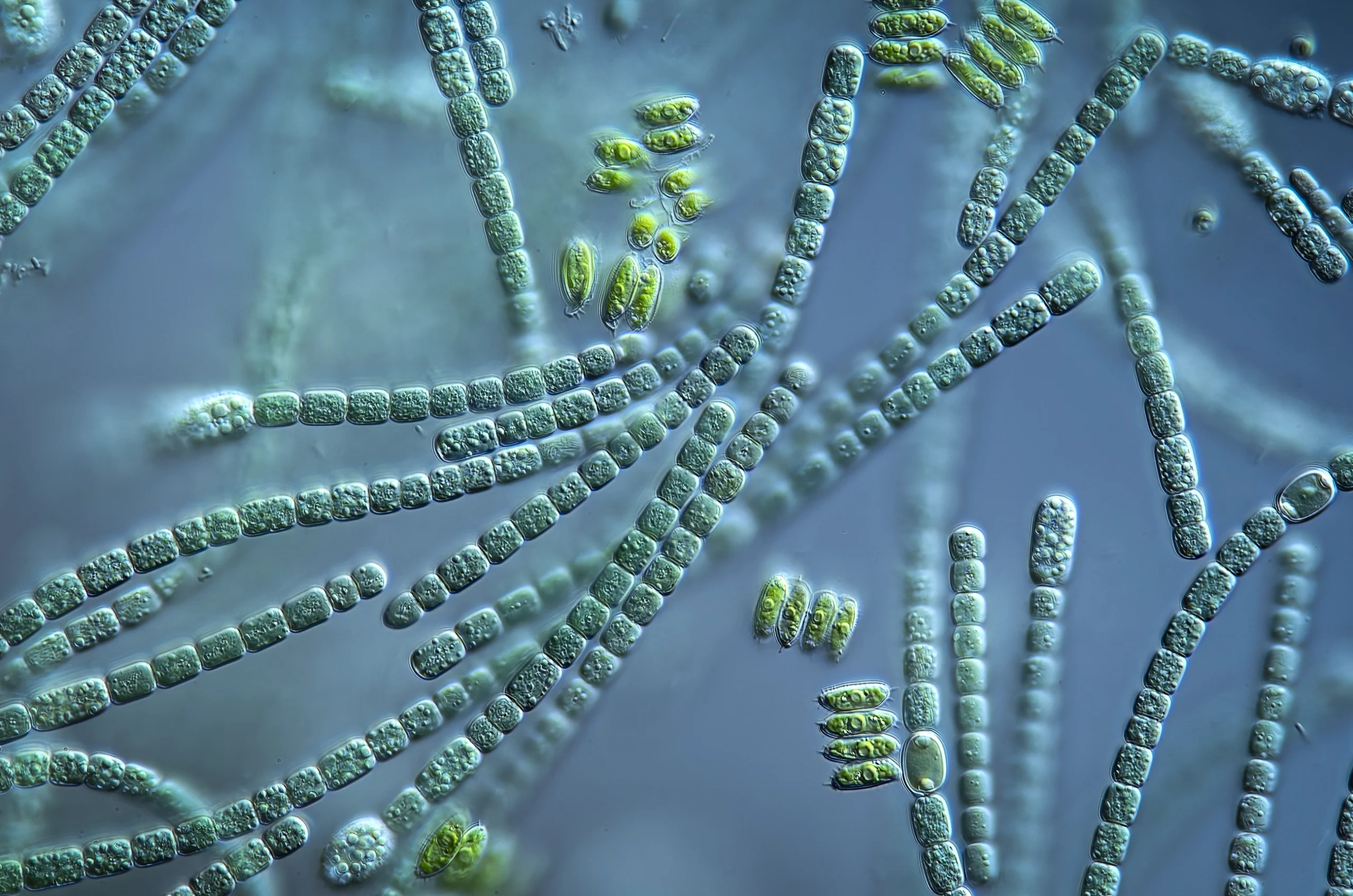
Cyanobacteria, a kind of phytoplankton.
Frank Fox/Science Source
A typical beneficial exchange is exemplified by photosynthetic diatoms and Roseobacter bacteria. The bacteria supply the diatoms with nutrients they can’t make themselves, such as vitamin B12, Amin said; the diatoms return the favor by feeding the bacteria dissolved organic carbon. These relationships can grow very close, with bacteria dwelling inside the diatom’s silicate shell or even coming inside the cell membrane and losing parts of their genome in the process.
On a larger scale, such microbial partnerships power the ocean’s ability to store carbon. At least half of global carbon uptake by photosynthesis occurs in the oceans, and 90% of that is done by phytoplankton; in 2023, the ocean absorbed an estimated 10.6 billion metric tons of carbon dioxide. When the remains of plankton settle on the seafloor, they are effectively sequestered in deep sediment. Therefore the productivity of plankton and their microbial partners determines how much carbon dioxide the ocean can remove from the atmosphere.
Warming oceans seem to test these kinds of relationships. For example, when seawater gets too hot, corals expel the algae that live in their tissues. Scientists, including Amin, are testing whether the same could be true for microbe-microbe partnerships. He is now studying bacterial microbiomes of corals in the Persian Gulf, one of the hottest seas in the world. If he can learn how these relationships have adapted to conditions that will soon become more widespread, then we’ll be better armed to preserve microbial partnerships throughout our warming world.