The Mystery of the Missing Multicellular Prokaryotes

How prokaryotic genomes respond to a drop in population size could be the barrier that has prevented the organisms from evolving complex multicellular forms.
Carlos Arrojo for Quanta Magazine
Introduction
Every organism visible to the naked eye is a mass of genetically identical cells. Each of these multicellular creatures started as a single cell that divided countless times to produce its body. And while each cell contains the same genome, they express their DNA in a variety of ways, giving rise to specialized cells and tissues that perform different roles, such as skin, liver or immune cells. This complex multicellularity has evolved independently in at least five lineages: animals, land plants, brown algae, red algae and fungi.
As different as these multicellular creatures might be, their bodies are all composed of the same type of cell — eukaryotic cells, which enclose their DNA in a nucleus and possess energy-producing mitochondria. The much older prokaryotic cells, which make up the vast kingdoms of bacteria and archaea and whose cells lack these features, never got complex multicellularity off the ground. They have evolved primitive forms of multicellularity, such as colonies of photosynthetic cyanobacteria. But there they stop. Even with a 1.5-billion-year head start on eukaryotes, prokaryotes never evolved this other way of living.
Why? “There’s never been a very satisfying hypothesis,” said Carl Simpson, a paleobiologist at the University of Colorado, Boulder. It’s possible that there is something exceptional about eukaryotic cells, such as their ability to build inner membranes, that makes it easier to achieve multicellularity. Or maybe the extra energy afforded by mitochondria helped eukaryotes kick-start the process. Or perhaps eukaryotes’ ability to reproduce sexually and regularly remix their genetic diversity got them over the hurdle. However, to some scientists these explanations can feel like just-so stories.
What if something much simpler and stranger is going on? In a recent paper in the Proceedings of the National Academy of Sciences, the biologists Emma Bingham and William Ratcliff of the Georgia Institute of Technology put forward a brand new idea, which they tested in a computational model. Bingham and Ratcliff suggest that the way prokaryotic and eukaryotic genomes respond to population size may make or break their chances of evolving multicellularity. It’s a fascinating hypothesis, and if further work bears it out, it could fundamentally change how scientists conceive of this transition and challenge a key assumption they make about evolutionary forces.
Population Pressure
Ratcliff has spent the last 15 years forcing single-celled eukaryotes to evolve multicellularity in the lab. He grew yeast, spun the cells in a centrifuge and then used only the very heaviest individuals, which had fallen to the bottom of the tube, to start the next day’s culture. Before long, this selection pressure encouraged a mutation that kept cells from separating, causing it to take over the population.
Snowflake yeast, as he and his colleagues called this form of the organism, creates branching structures so enormous that they’re visible to the naked eye. His team now studies the conditions under which this rudimentary multicellularity arises, in hopes of understanding the evolutionary pressures and benefits that, 1.6 billion years ago, may have influenced the development of multicellular life.
Over the years, as Ratcliff watched his snowflake yeast form bigger and bigger bodies, he pondered a basic fact: Once yeast made the leap to multicellularity, what could have been a million one-celled organisms instead became one individual. An unavoidable side effect of the evolution of multicellularity is that population size crashes. “Nothing kills effective population size like multicellularity,” Ratcliff said.
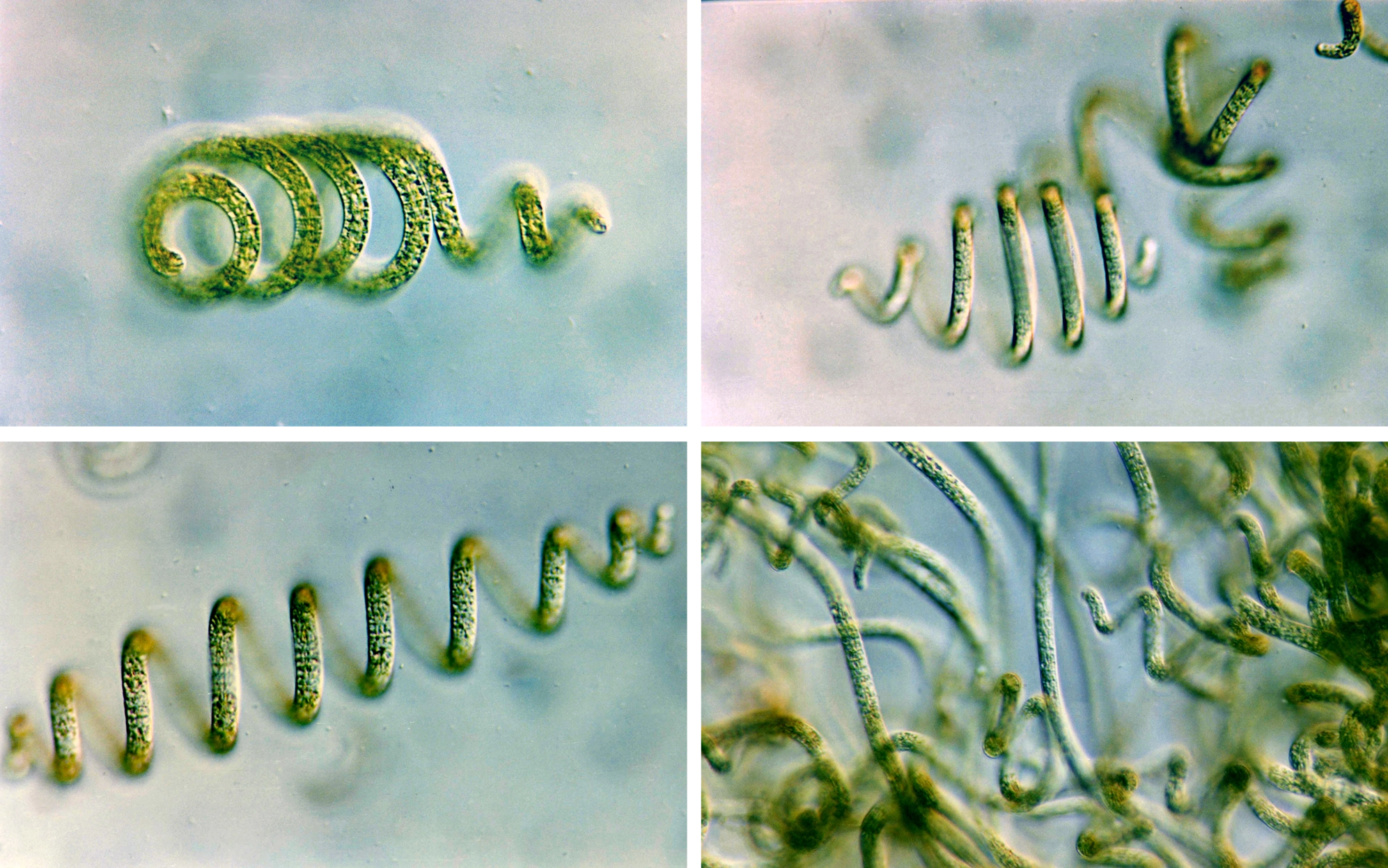
Arthrospira, a genus of cyanobacteria, has evolved a primitive form of multicellularity. Many cells grow alongside one another in coiled colonies — but they have never managed to specialize into varied cell types or tissues.
C. Sili, Giuseppe Torzillo, and Avigad Vonshak
One day, he was discussing this phenomenon with Bingham and other graduate students when they realized how it might connect to the mystery of the missing multicellular prokaryotes. In 2007, Michael Lynch, an evolutionary biologist at Arizona State University, had pointed out a flaw in the way many people were thinking about evolution. It is easy to assume that evolution is synonymous with natural selection, the force that weeds out less advantageous forms of genes and promotes the survival of the fittest. But there are other forces at work, often at odds with natural selection. One of them is genetic drift, which introduces random shifts in genetic variation across generations. And in small populations, genetic drift is more influential than many people realize.
In large populations, the effects of genetic drift are minimal. When many individuals breed, their genes get randomly shuffled and re-sorted in the next generation, with only average odds of being passed along. Unless natural selection steps in to kick some traits out of the gene pool or to privilege beneficial new mutations, the genetic profile of the population stays about the same.
But in small, isolated populations, chance is king. One individual will sometimes produce more offspring for random reasons, not because it’s a fitter organism than the others. Within a single generation, that individual’s DNA — good genes and bad genes, it doesn’t matter — can end up in a much larger share of the population, perhaps even the majority of it. Traits that might normally be rare or disadvantageous can then get entrenched in the isolated group and evolve further.
Ratcliff thought of all this in a flash, and then remembered something else: Population geneticists have reported that the genomes of eukaryotes and prokaryotes seem to respond differently to strong genetic drift. In a small population, eukaryotic genomes tend to balloon, duplicating genes and adding more DNA. The genomes of complex multicellular eukaryotes today, in fact, are massive and full of repeating sequences, on-off switches and intricate controls on gene expression, features that seem to enable some of the complexity seen in eukaryotic life. “[Lynch] very beautifully showed, especially for eukaryotes, that relaxation of selection could be important for getting the features of complex genomes that we know and love,” Ratcliff said.

Stromatolites, a sizeable form of primitive multicellularity, emerged in bacteria at least 1.25 billion years ago — and never evolved greater complexity. The microorganisms live colonially and generate microbial mats, which glue together sediments to form boulderlike structures like these found in western Australia.
MaXPdia/iStock
The genomes of prokaryotes, however, tend to collapse in size in the face of genetic drift, as suggested by work done by Howard Ochman of the University of Texas, Austin and Louis-Marie Bobay, now at North Carolina State University. It’s not clear why, but it may be because gene regulation in prokaryotes is much less complex: All of their genes are transcribed. Deleting DNA could be their way of dealing with less-than-ideal genes taking over under genetic drift, speculated Simpson, who was not involved in the new research. Maybe jettisoning chunks of the genome is a defense against the random effects of drift.
It wasn’t clear if all prokaryotes do this, Ratcliff knew. But if, in a pinch, ancestral prokaryotes got rid of DNA instead of accumulating it, could they have had trouble assembling the toolkit to make a transition to multicellularity?
Ratcliff and Bingham, who is a computational biologist, thought about the ramifications. In effect, the evolution of multicellularity can squeeze what was once many organisms into a smaller population; there might be a lot of cells, but they’re all the same organism. Under this population pressure, prokaryotes and eukaryotes might respond differently — the former in a way that creates barriers to evolving multicellularity, and the latter in a way that accelerates it.
In that case, the transition might have fueled, and been fueled by, eukaryotes’ tendency for genome expansion. In other words, the ability to make the transition to multicellularity might come down to how a population handles its genome in a tight spot. Ratcliff and Bingham wondered if they could find a way to test that idea.
A Toy Model
Ratcliff and Bingham were already aware that multicellularity seems to coincide with increased genome size. “We know from the comparative record that pretty much all multicellular lineages have bigger genomes than their single-celled counterparts,” Ratcliff said. But they wanted to take a closer look.
As a test case, they examined the genomes of cyanobacteria, which have evolved many forms of primitive multicellularity. Ratcliff and Bingham discovered that the more features of multicellularity a given lineage of cyanobacteria had, the larger its genome. This provided support for a link between genome size and multicellularity.
Then, to examine their hypothesis more directly, Ratcliff and Bingham created a highly simplified computational model. In the model, populations of prokaryotes and eukaryotes were under pressure to develop into multicellular organisms of different sizes and accumulate a larger genome. The researchers programmed one group, standing in for eukaryotes, with a tendency to expand its genome; the other, standing in for prokaryotes, shrank it. Even as the organisms grew larger, the eukaryotes were always able to grow their genomes substantially and were rewarded for it. Below a certain size, however, the prokaryotes ran into a wall and couldn’t make their genomes larger, no matter how much they were rewarded.
The model is simple and limited, the researchers state. Still, it suggests that this difference in genomic processes could have significant effects.
Their new hypothesis is striking, Simpson said, because it jibes with other observations about how multicellularity works. Complex multicellular organisms have radiated into many different species. Perhaps that’s a result of additional DNA piling up, providing the raw materials for innovation.
It also connects to the procedures that complex multicellular eukaryotes use to develop different cell types from the same set of genes. They manage to have tissues as different as the liver and the lungs by turning genes on and off in different cells. Perhaps prokaryotic organisms that didn’t start out with the ability to control their DNA that way would have struggled to make the leap.
Crucially, whenever eukaryotes did manage to put together a toolkit to become multicellular, they would not have to fight a genomic process to keep it, Simpson said. “But prokaryotes have to fight it all the time no matter what.”

Myxobacteria, sometimes called slime bacteria, display an unusual primitive multicellularity. When nutrients are scarce, tens or hundreds of thousands of the bacteria aggregate into colonial fruiting bodies, which increase their survival chances if conditions improve. Myxobacteria also have large genomes: One species has the largest known genome of any bacterial species, more than 16 million nucleotides long.
Alison Pollack
However, not every modern eukaryote expands its genome when population sizes are small. Fruit fly genomes, most notably, tend to shrink. To advance the new hypothesis, future research should address how and under what circumstances it might happen, said Eörs Szathmáry, a theoretical biologist at Eötvös Loránd University in Budapest who studies major transitions in evolution and was not involved in the project. “This difference [between eukaryotes and prokaryotes] is poorly understood.”
Lynch, whose work inspired Ratcliff and Bingham, flagged another of the hypothesis’s assumptions that could use fleshing out: It’s not 100% certain that a larger genome does mean the ability to do more interesting things.
Still, Szathmáry, who wrote a commentary on the paper for the journal, said the idea is appealing. He likes the cleverness and simplicity of what it suggests. “These big evolutionary changes — what we call evolutionary transitions — these are not the easiest problems to solve. … Difficult questions need new ideas,” he said. “It’s detrimental to be too dogmatic when these profound questions arise, because we want to collect pieces of the puzzle and not kill ideas in their infancy.”
The hypothesis challenges the common assumption that natural selection is the only force at work in evolution. “[It’s] a big problem we have in evolutionary biology,” Lynch said: Biologists feel that they must account for organisms’ modern traits by saying they were selected for, when, in fact, chance — such as chance driven by strong genetic drift — might have played a significant role in a trait’s rise. “It really cheapens the field, and it has reduced our ability to think more broadly about what might have happened,” he said.
Lynch feels that Ratcliff and Bingham laid out their ideas and caveats well. “This is how we proceed in developing theory,” he said. “We start with some assumptions. If you state your assumptions clearly, people can reevaluate as more data comes in.”
Now that the idea is out in the world, Ratcliff hopes that others will be inspired to test it. And through it, he sees ways into other interesting questions about how genomes work. Could eukaryotes’ tendency toward expansion be linked to the transposable elements — the leaping, self-duplicating bits of DNA — that have infested eukaryotic genomes for eons? “I suspect that a lot of eukaryotic cell biology is driven by the ability to deal with parasitic elements,” he said. These invaders, which now make up a substantial portion of every eukaryote’s genome, may have helped set the stage for multicellularity, he said.
Ratcliff knows that his hypothesis implies a different reality than the one most people who think about multicellularity for a living have inhabited until now. The field has historically emphasized the idea of multicellularity as an outgrowth of eukaryotes’ special abilities rather than as a result of how their genomes work.
But the idea is powerful because it invokes an alternate world, one where, long before the origin of plants, great kelp-like forests made of cyanobacteria could have been swaying in the ocean. “There’s no reason why a cyanobacterium couldn’t evolve to be a seaweed, in my mind,” Ratcliff said — except, perhaps, for a quirk of their genomes.